US FDA User Fees
Are you sure you'd like to remove this alert? You will no longer receive email updates about this topic.

CDER, CBER Not Seeing Hiring Slowdown Despite US FDA Warnings
FDA officials have said hiring could be slowed if an inflationary pay increase is not included in the agency budget, but CDER and CBER continue to add staff at a steady pace.

Parallel Scientific Advice: Is The EMA User Fee Impacting Interest?
EMA charges participants to receive scientific advice through the fledgling program, unlike the US FDA, which may not fit the budgets of some complex generic sponsors. At the same time, sponsors also may simply not be aware the program exists yet.

CDER, CBER Post FY 2023 Employee Gains, But Behind User Fee Hiring Goals
US FDA’s drug center reported a net increase of more than 300 employees in FY 2023, while the biologics center saw an overall increase of more than 30.

Congress Gives US FDA Austere Budget But Seeks Boosts On Inspections, IT, And ALS Activities
The minor cut would be masked by user fee revenue increases, but still would be the first time in at least a decade that agency's budget authority has dropped.
EMA's New Cost-Based Fee Structure Gets Final Go-Ahead From EU Ministers
EFPIA has welcomed the adoption of the new regulation, which will take effect from January 2025. However, it cautioned that the EMA and the national agencies needed to have the necessary resources to do their job and that the new fee structure should be supported by revisions to the EU pharmaceutical legislation.

Pink Sheet Podcast: US FDA Adcomm Schedule, CMS And Drug Importation, Missed User Fee Goals
Pink Sheet reporters and editors discuss the nearly wide open 2024 FDA advisory committee calendar, CMS’s impact on Florida’s drug importation plan, and the FDA’s many missed user fee goals in 2023.

Pink Sheet Podcast: Looking Back And Looking Ahead: Key US and EU Legislation
Pink Sheet reporter and editors discuss key US and EU legislation that impacted 2023 and how it also could affect 2024, including EU legislative reform, US Medicare drug price negotiation, and the US Food and Drug Administration user fee reauthorization.

ANDA Sponsors Need US FDA Permission To Avoid Last-Minute Label Update Delays
Under changes in the 2022 appropriations bill, generic drug applications can be approved even if a last-minute labeling update is needed, but sponsors risk product misbranding if the CBE is late.

US FDA’s No Recording Meetings Rule Already Appears Flexible
At least one sponsor has been recording meetings for several years, suggesting others could receive permission, but the decision comes with risks as well as benefits. As the agency again expands options for in-person meetings, sponsors may want to reflect on what works best for them.

Minutes Matter: Why The US FDA Refuses To Record Formal Meetings
Given confusion sometimes emerges about the content of formal meeting minutes, recording the meetings would seem like an easy solution, but also could muddy the administrative record.
Generics Review Data From US FDA Reveals Positives, Negatives For Industry
While a decline in complete response letters in FY 2023 is good, the continued drop in ANDA submissions could be a bad sign for the industry’s long-term health, a former FDA official worries.

US FDA Lab Test Rule Cites Explosion In Companion Diagnostics, COVID Lessons
FDA launches what it hopes will the final push to reform the regulatory oversight system for lab-developed tests by proposing a relatively rapid transition period to require essentially all LDTs to comply with the existing regulations for in vitro diagnostics. Legislation, litigation, and industry negotiation will likely shape the final product.

US FDA’s Standard Application Assessment Makes A Comeback
After years where many more applications received priority assessments than standard assessments, in FY 2022 the difference narrowed.

Preparing For A Shutdown: US FDA To Retain 81% Of Workforce, Thanks Mostly To User Fees
However, user fee carryover balances will not last forever and service cuts are inevitable if a shutdown lingers for several weeks.

EU Bodies Agree On Ways To Future-Proof EMA Funding System
EU ministers say that implementing an appropriate fee structure that is more in line with actual costs will promote innovation in the pharmaceutical sector while ensuring “fair access” to safe and effective medicines for patients.
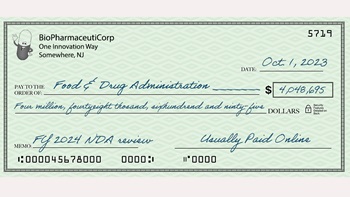
The $4 Million NDA: US FDA User Fees After 30 Years
The cost of filing a New Drug Application with the US FDA has pushed above the $4 million mark. That is a remarkable rate of increase from the original $100,000 fee set in 1992 – but it does buy a lot more.
You must sign in to use this functionality
Authentication.SignIn.HeadSignInHeader
Email Article
All set! This article has been sent to my@email.address.
All fields are required. For multiple recipients, separate email addresses with a semicolon.
Please Note: Only individuals with an active subscription will be able to access the full article. All other readers will be directed to the abstract and would need to subscribe.