Pricing Debate

Quick Listen: Scrip's Five Must-Know Things
In this week's podcast edition of Five Must-Know Things: Teva’s immunology biosimilar launches; Intra-Cellular’s promising depression data; Phase III sleep apnea win for Lilly’s tirzepatide; an interview with Viking’s CEO; and China looks to define innovative drugs.

Medicare Reimbursement For Part B Drugs Is 48% Above 340B Prices To Hospitals, MedPAC Finds
New study updates past commission analyses and highlights the significant margin between Medicare payments for drugs and the prices paid by 340B-eligible providers.

Japan Regulatory Update: Revised Law Widens RWD Scope, Price Revisions/Listings
Japan now allows pseudonymized personal data for medical use under a licensing system for wider use of real-world data. Meanwhile, a national cost-effectiveness assessment scheme has slashed reimbursement prices for Lagevrio and Kerendia, and Alexion’s Voydeya has been added to the reimbursement tariff.

Medicare Reaffirms Faith In Formulary Review Process Ahead Of Part D Changes
Dramatic transformation in the US Medicare Part D benefit design does not require any changes to CMS’ overall approach to reviewing formulary submissions from private drug plan sponsors, the agency says in its final guidance implementing the design changes for 2025.

Califf Suggests Congress ‘Tighten Up Laws’ To Address Orange Book Abuses
US FDA Commissioner repeats longstanding FDA policy that the agency doesn’t have much power to address frivolous patent listings in the Orange Book, but seems to support Congress providing more authority.

Quotable: Top Experts On Policy Hot Topics
The Pink Sheet highlights recent comments and insights from pharma officials and executives on key issues we are covering.

Cost-Based Pricing: The Real Threat Hiding In Latest US Drug Price Headlines
Bernie Sanders is back at it, getting headlines for demanding immediate price cuts to Novo’s blockbuster GLP-1 brands. But behind the familiar rhetoric is a newer threat: academic research on cost-based pricing that could one day feed into the emerging Medicare ‘negotiation’ system.

Obesity Drugs Cost Concerns Not Yet Impacting HHS Risk Adjustment Models
Individual and small group plans in the commercial insurance market are not seeing ‘a large enough change in clinical indications or practice patterns to warrant a change to the current mapping of GLP-1 drugs,’ final rule states.

Biden vs Trump On Drug Pricing And Unfinished Business
While more significant reforms are less likely under a second Biden term unless Democrats control both chambers of Congress, former President Trump could look to put his stamp on drug pricing reform by resurrecting one of his former policies involving international reference pricing and Medicare Part B drugs.

Bernie Gets Mostly Pharma-Friendly Drug Pricing Results
Changes to inhaler pricing may be more ‘business as usual’ for the drug industry than the Vermont senator wants to let on. FTC, meanwhile, is keeping the patent pressure on Teva, the only inhaler manufacturer to not act following Sanders’ investigation.

Older Medicines Rebate Will Test UK’s New Pricing & Access Scheme
As the UK government implements a new rebate system for older medicines as part of the new pricing deal, industry trade bodies and experts suggest that exceptions will have to be made, such as higher prices or lower rebate levels for specific products.

Industry To Scrutinize Proposals On Updating UK Statutory Pricing Agreement
The UK government is consulting on updates to the UK’s statutory pricing scheme to bring it further into line with the voluntary pricing scheme.

AstraZeneca Joins The $35 Club With Cap On Inhaler Copays
The British drug maker’s move follows that of Boehringer Ingelheim earlier this month, which itself followed increasing political scrutiny on out-of-pocket costs.

AstraZeneca Joins The $35 Inhaler Club; Medicare Patients Waiting On Congress For Membership
AZ’s move follows Boehringer’s announcement earlier this month and parallels what insulin sponsors did – but almost in reverse. For inhalers, political pressure on out-of-pocket costs has produced copay caps in the commercial market first, not Medicare.
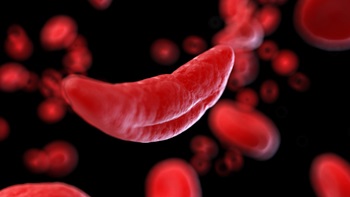
Vertex Resolves To Reverse English Funding Rejection For CRISPR Sickle Cell Gene Therapy
The health technology assessment institute, NICE, is not yet ready to recommend Casgevy for sickle cell disease and says it wants more data. Meanwhile, an access agreement relating to the treatment’s use for transfusion-dependent β-thalassemia is making progress in England, as are reimbursement talks for SCD in other European countries.
With Reform Efforts Persisting, PBM Trade Association Sets Lobbying Record
Infographic details who gave what to whom as all segments of the US healthcare industry gear up for the presidential election cycle.
You must sign in to use this functionality
Authentication.SignIn.HeadSignInHeader
Email Article
All set! This article has been sent to my@email.address.
All fields are required. For multiple recipients, separate email addresses with a semicolon.
Please Note: Only individuals with an active subscription will be able to access the full article. All other readers will be directed to the abstract and would need to subscribe.