Interviews
Are you sure you'd like to remove this alert? You will no longer receive email updates about this topic.
A selection of in-depth interviews and profiles of policymakers and industry executives.

Woodcock Takes On Rare Disease Challenges In Retirement, Keeps FDA, Industry At Arm’s Length
Recently retired US FDA Principal Deputy Commissioner Janet Woodcock will be advising the Haystack Project, with the goal of helping rare disease organizations encourage creativity in drug development programs without jeopardizing regulatory success, Woodcock told the Pink Sheet in an interview.

EU Needs Market Access Improvements Beyond New HTA Regulation
While the EU Health Technology Assessment Regulation could reduce divergence in reimbursement decisions made across member states, many national-level HTA hurdles and challenges will remain, market access experts from EFPIA say.

‘Unbalanced’ EU HTA Timelines Exacerbated By Rare Disease & Cancer Drugs
As it stands, drugmakers will have just 90 days to prepare their dossiers for EU-wide joint clinical assessments under the new Health Technology Assessment Regulation. Market access experts from EFPIA tell the Pink Sheet that this short deadline could delay patient access to complex medicines, such as innovative cancer drugs.

Estonian Biobank’s Personalized Medicine Project To Unlock New Insights For Drug R&D
A new center for personalized medicine in Estonia will see PacBio’s long-read whole genome sequencing technology used to unlock new information about how genetics impact patients’ drug responses, experts from the project tell the Pink Sheet.

Breaking Barriers: Japan's PMDA To Open First Global Office, In D.C.
Through a new full-time office in the US capital, Japan's drug regulator aims to provide essential regulatory information to help US bioventures enter the Japanese market at an early stage.

'Don't Compromise The Pharma Innovation System,' Says Outgoing IFPMA Head Thomas Cueni
Thomas Cueni is stepping down after seven years at the helm of the International Federation of Pharmaceutical Manufacturers and Associations. In an interview with the Pink Sheet ahead of his retirement, he reflects on the proposed pandemic treaty, what still needs to be done to ensure equitable access to medicines, the importance of tackling AMR, moves towards regulatory reliance – and what his post-IFPMA life might look like.
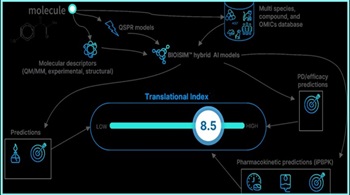
When Using Artificial Intelligence In Pharma R&D, Start With Identifying Problem To Solve
VeriSIM uses generative AI for questions such as changing a drug molecule’s chemistry and machine learning to better predict potential biological implications, says CEO Jo Varshney.

How The EU & UK’s Contrasting Approaches To AI Regulation Could Impact Pharma
While EU preparations are underway to introduce strict legal requirements for all AI systems, the UK has doubled down on its flexible, non-regulatory framework. In this second of a two-part article, a lawyer explains the pros and cons of each for pharma and medtech firms.

‘A Maze Of Rules’: EFPIA On EU’s Proposed Health Data Sharing Law
While some progress has been made in strengthening the proposed European Health Data Space during legislative negotiations, further amendments must be made to protect trade secrets and avoid fragmentation, pharma industry federation EFPIA says.
EMA’s Cancer ‘Pathfinder’ Project Targets Conditional Approvals, Endpoints & RWE
This second of a two-part article on the EU’s “Cancer Medicines Pathfinder” initiative looks at the trade-off between the benefits and risks of conditional drug approvals and differing perceptions of progression-free survival as a validated endpoint in cancer drug trials.

Blue Shield Of California’s Pharmacy Benefit Overhaul Could Still See Boost From Transparency Legislation
The payor’s multiyear plan to ditch the traditional PBM model and use multiple vendors has drawn a lot of skepticism from other actors in the health care system, but Blue Shield is hoping that it can help give policymakers some momentum, VP Lum tells the Pink Sheet.

EU Rare Disease Policy Needs To Help Revive Abandoned Candidates & Repurpose Existing Drugs
The EU must adopt a more comprehensive policy on rare diseases to increase the number of orphan drugs and address access challenges, says EURORDIS’ chief executive Yann Le Cam.

‘We Did Too Good Of A Job’ On Lowering Prices: Sandoz’s Haruvi Discusses Generic Sustainability
As Hatch-Waxman celebrates its 40th anniversary, the chair of the US generic trade association talks about striking the right balance on supply and pricing, preventing shortages, and improving Medicare price negotiations.

Creating A ‘Critical Mass’: Outgoing EURORDIS Chief On 25 Years Of EU Orphan Disease Policy
As Yann Le Cam prepares to step down from his role as leader of the EU network of rare disease patient organizations, EURORDIS, he reflects on the organization’s role in almost three decades of major changes to Europe’s orphan disease and drug policy.

Pazdur On Accelerated Approval: FDA Needs To Explain Why It Does Not Always Seek Withdrawal When Trials Fail
Knee-jerk reaction when a confirmatory trial fails is that the drug should be withdrawn, but the agency must undertake a more nuanced evaluation and do a better job explaining it to the public, OCE Director Richard Pazdur says; Pazdur and Project Confirm lead Gautam Mehta spoke with the Pink Sheet about dangling indications, FDORA reforms and the withdrawal process.

Confirmatory Trials: Flexibility In Timing Hinges On Accelerated Approval Indication Size – FDA’s Marks
CBER director suggests more flexibility on confirmatory study timing is warranted for accelerated approvals in rare diseases and even in some more common scenarios such as infectious disease outbreaks, although a ‘relatively stronger approach’ will apply to most large indications.
You must sign in to use this functionality
Authentication.SignIn.HeadSignInHeader
Email Article
All set! This article has been sent to my@email.address.
All fields are required. For multiple recipients, separate email addresses with a semicolon.
Please Note: Only individuals with an active subscription will be able to access the full article. All other readers will be directed to the abstract and would need to subscribe.