Patents
Lupin became the latest ANDA sponsor to settle patent-infringement litigation over Harmony Biosciences’ Wakix (pitolisant hydrochloride), which has been touted as a potential blockbuster.
In a somewhat surprising move, President Trump’s Federal Trade Commission is continuing a crusade to delist improper listings from the FDA’s Orange Book. Law firm Polsinelli’s chair Chad Landmon discussed the impact of the move on the generic drug industry.
Experts from EUCOPE explain why the Council of the EU’s position on the proposed overhaul of the general pharmaceutical legislation could offer more predictability for companies than the commission’s initial offering.
Acadia’s Nuplazid for hallucinations and delusions associated with Parkinson’s disease psychosis appears safe from generic competition until well into the next decade, following a favorable infringement and validity decision by a US district court.
The verdict by the Unified Patent Court in the dispute between Sanofi/Regeneron Pharmaceuticals and Amgen explains what companies should look out for when deciding whether infringement has taken place when it comes to second medical use patents.
Teva was forced to delist its ProAir HFA inhaler patents from the FDA’s Orange Book by mid-March after the Federal Circuit denied its petition for en banc rehearing. Will the Supreme Court listen?
The FDA Commissioner nominee also commented on trial diversity, the role of public comment and expedited biosimilar reviews during his Senate confirmation hearing.
The International Generic and Biosimilar Medicines Association has introduced its first global intellectual property and competition report, addressing the urgent need to reform current regulations. But, in a bid to increase access to medicines across the world, did it stop short at just IP and patents?
US pharma leadership is painting its mission, particularly its goal to end Medicare price controls, as patriotic and essential to America’s global dominance over China, a move that seems designed to align with President Trump’s priorities.
While the UK Court of Appeal’s decision in Merck Serono v Comptroller-General will be unwelcome by innovators, it provides much-needed certainty for companies seeking – or seeking to invalidate – supplementary protection certificates based on marketing authorizations for new uses of known active ingredients.
Europe’s public-private Innovative Health Initiative is seeking contenders for two research projects relevant to pharmaceuticals, one relates to the incoming European Health Data Space Regulation and the other focuses on reducing exposure to medicines containing per- and polyfluoroalkyl substances.
The US PTO faces criticism after withdrawing a proposed rule intended to address double-patenting by changing terminal disclaimers.
Removing the patent-protected indication from the label of Norwich Pharmaceuticals's proposed generic for Xifaxan did not convince the US Supreme Court to hear its petition to review a case from Bausch Health that blocked approval until 2029.
The new court ruling could enhance the appeal of the Unified Patent Court for enforcing European patents.
Companies are being encouraged to look at whether they will be impacted by new UK rules on supplementary protection certificates that come into effect from the beginning of next year.
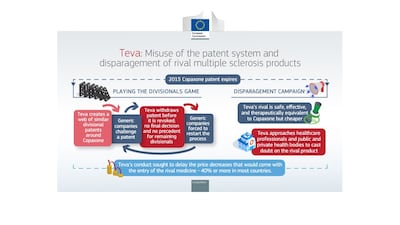
Teva has been fined €463m – just over half a billion US dollars – over a breach of EU antitrust rules, after the European Commission found that it abused its dominant position to delay competition to Copaxone, including by misusing the patent system and disparaging rivals. The firm has strongly disagreed with the decision – which is claims is “legally untested” and “not supported by the facts” – and says it will appeal.
A ruling by the Court of Justice of the EU has produced a clear definition of what constitutes the “first” marketing authorization when companies apply for SPCs on pharmaceutical products.
Divisional patents remain a major barrier to market entry for generics in Europe, heard attendees to Medicines for Europe’s legal affairs conference in Dublin earlier this month.
US Federal Trade Commission says the policy would enhance its ability to detect reverse payment settlements between pharmaceutical companies that raise antitrust concerns.
Industry groups say the initiative will undermine innovation and the competitiveness of European companies, impeding Europe's ability to tackle future crises effectively.