Mylan’s Generic Advair Approval Showcases US FDA Efforts To Boost Complex Products
Executive Summary
Commissioner Gottlieb previews actions agency intends to take in 2019 to smooth the regulatory and scientific pathway for generics of complex drugs, including draft guidance with recommendations on establishing active ingredient sameness.
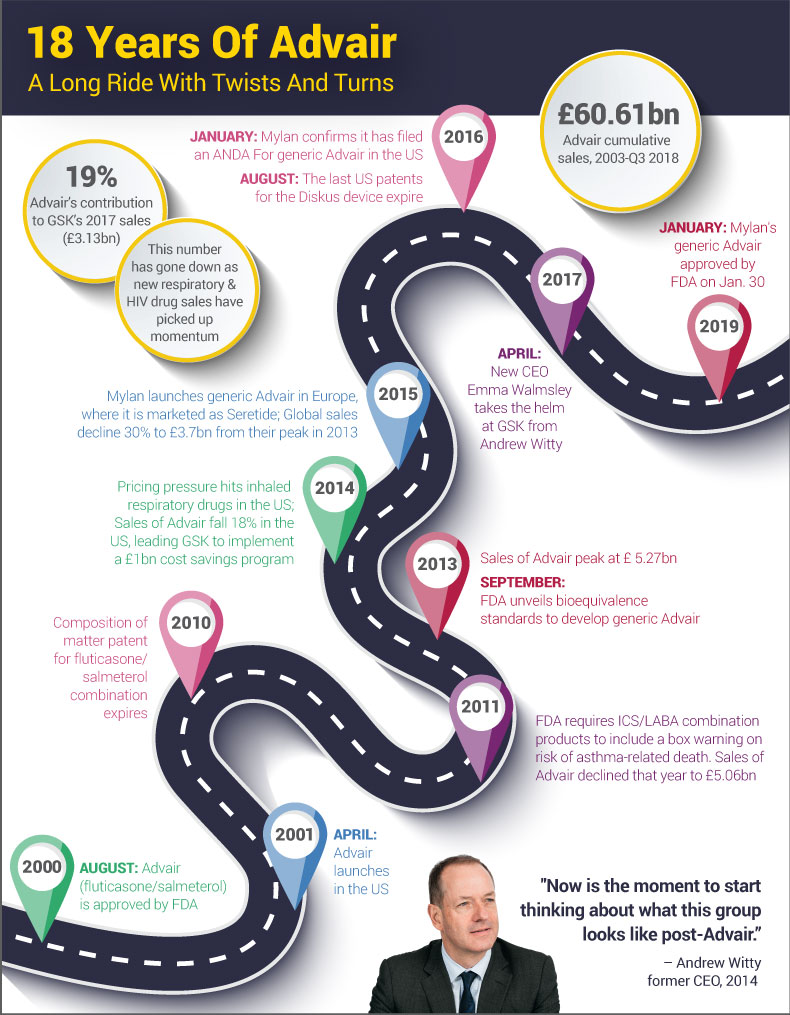
Mylan NV became the highest profile industry beneficiary of the US FDA’s push to bring more complex generics to market with the agency’s Jan. 30 approval Wixela Inhub, the first generic version of GlaxoSmithKline PLC’s hard-to-copy Advair Diskus (fluticasone propionate and salmeterol inhalation powder).
Mylan gained FDA approval for three strengths of the inhaled treatment for asthma and chronic obstructive pulmonary disease: fluticasone propionate 100mcg/salmeterol 50mcg, 250mcg/50mcg and 500mcg/50 mcg.
"Today's approval of the first generic drug product for one of the most commonly prescribed asthma and COPD inhalers in the US is part of our longstanding commitment to advance access to lower-cost, high-quality generic alternatives," Center for Drug Evaluation and Research Director Janet Woodcock said in an agency press release.
"People living with asthma and COPD know too well the critical importance of having access to the treatment they need to feel better,” Woodcock said. “Today's approval will bring more competition to the market which will ultimately benefit the patients who rely on this drug."
The approval comes more than eight years after GSK’s composition of matter patent on the fluticasone/salmeterol combination expired, and more than two years after the last patents on its Diskus inhalation device expired. (See timeline, below.)
Mylan submitted its abbreviated new drug application (ANDA) more than three years ago but had to overcome two complete response letters. (Also see "Branded Advair Breathes Another Day; Mylan Says A CRL Is On The Way For A Generic" - Pink Sheet, 13 Jun, 2018.)
With the approval, Mylan beat out Sandoz International GMBH and Hikma Pharmaceuticals PLC, which also were dealt CRLs on their Advair ANDAs. (Also see "US FDA Releases Slew Of Guidances On A Fell Day For Complex Generics" - Pink Sheet, 8 Feb, 2018.)
The complexity of GSK’s drug/device reference product has proven challenging for generic drug sponsors to copy to FDA’s satisfaction despite the agency’s release in 2013 of draft guidance on bioequivalence recommendations, formulation and device considerations for dry powder inhalers containing fluticasone propionate and salmeterol xinafoate.
"The FDA recognizes challenges companies face when seeking to develop hard-to-copy complex generics, such as drug-device combination products, including when the drugs are incorporated into inhalation devices like this," said Anna Abram, FDA's deputy commissioner for policy, planning, legislation and analysis. "We are committed to advancing new guidance for sponsors to make the development of generic versions of complex products more efficient, and we're prioritizing review of many applications covering proposed generic complex products for which a generic has not yet been approved."
Additional Guidance Coming In 2019
Promoting generic competition to complex, off-patent drugs has been a cornerstone of the agency’s efforts to reduce drug prices under FDA Commissioner Scott Gottlieb.
At his confirmation hearing, Gottlieb suggested that both administrative steps and legislative changes may be necessary to speed the approval of more complex generics. (Also see "Complex Generics: Gottlieb Eyes FDA Policy Changes To Speed Approvals" - Pink Sheet, 5 Apr, 2017.)
The second iteration of the Generic Drug User Fee Act created a new meeting process by which companies can seek scientific advice from the agency on their development of a complex generic. (Also see "Complex ANDAs To Be Allowed Pre-Submission Product Meetings" - Pink Sheet, 24 Oct, 2016.)
In January 2018, FDA convened a workshop on bioequivalence assessments for generic orally inhaled and nasal drug products. (Also see "Complex Generics: US FDA Still Wants Comparative Clinical Endpoint Studies For Inhaled, Nasal Drugs" - Pink Sheet, 16 Jan, 2018.)
In October, FDA released a series of guidance documents on developing generics of transdermal and topical delivery systems, another category of complex products. (Also see "Complex Generics: US FDA Heeds Some, But Not All, Of Industry’s Calls For Flexibility On Transdermal Products" - Pink Sheet, 9 Oct, 2018.)
In a statement separate from the FDA press release announcing the Mylan approval, Gottlieb previewed some the policy steps the agency intends to take in the coming year to advance complex generics.
“We intend to issue additional guidance documents for developing specific complex generic drugs, as well as address categories of complex drugs that are hard to copy because of their complex formulation or mode of delivery,” he said. “This will include the publication of a series of guidances to address regulatory and scientific challenges that make it generally more difficult to develop complex generics.”
“As part of this, we intend to issue draft guidance with recommendations on establishing active ingredient sameness,” Gottlieb said.
“In addition, we're going to help advance the development of new analytical tools and in vitro tests that may provide additional accurate, sensitive and reproducible tools to support approval of complex generic drugs. Better tools can reduce complex generic drug development time and cost and can inform regulatory decisions.”
The new policy efforts are aimed at ensuring the agency provides as much scientific and regulatory clarity as possible, the commissioner said. “This focus is critical because, first and foremost, these drug products provide important therapies to patients. We believe that they're also becoming increasingly important to the economic stability of the generic drug industry. Being able to ‘genericize’ a complex medicine can be a high-value opportunity for a generic drug developer.”