Random Checks Now Routine: China To Inspect Your Factories Near And Far
Executive Summary
An officially released new regulation in China legalizes overseas inspections of drug and device makers, making the risk-based inspections routine tasks that could also expand to manufacturers' R&D partners and third-party suppliers of ingredients, excipients or packaging materials.
A new regulation on inspections of foreign manufacturing facilities issued by China’s National Medical Product Administration (NMPA) a day after Christmas has many implications for drug and device makers who are already marketing products on, or are eyeing, the Chinese market.
The scope of the inspections includes the R&D and manufacturing process of all drugs and devices that are currently marketed, or intended to be marketed, in China, the aim being to ensure that overseas sites supplying China adhere to Chinese quality and safety standards.
The backdrop to the new move is a steady increase in such overseas inspections by Chinese authorities over the past few years, noted Ropes & Gray’s China healthcare partner Katherine Wang; initiated in 2011 for drug manufacturers, these have since been extended to medical device makers.
So far, China's Center for Food and Drug Inspection (CFDI) has increased the number of checks from an initial seven products in 2011 nearly five-fold, to 33 products in 2018, with a total of 131 drugs having already gone through the process. The center extended the inspections to medical devices in 2015, and has so far looked at 90 products in this field.
Number Of China Overseas Inspections
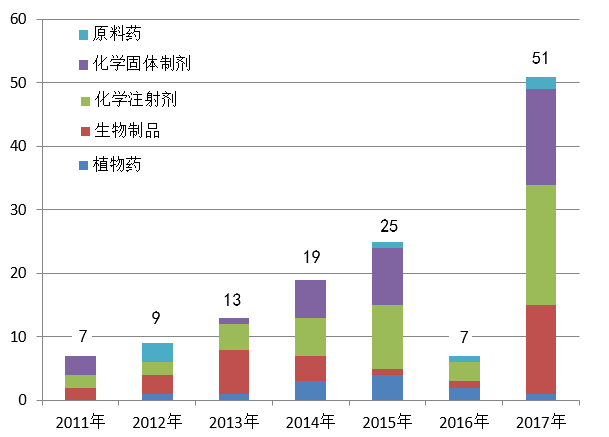
(Purple is facilities for oral tablets, green is injectables, red biologics, and blue plant-derived medicines. Source: CFDI)
Out of 51 products inspected during 2017, chemical drugs accounted for 36, and nine were deemed to have failed due to GMP non-compliance, noted the CFDI in its annual report. While Europe and North America are the major regions for the inspections so far, India is increasingly becoming another hot spot for the checks, the center said.
“The inspections covering products' complete cycle is becoming more routine, and drug inspections are increasingly becoming one of the most important technical tools for regulators," noted CFDI director Dong Jiangping in early 2018.
Risk-based Inspection
The new regulation outlines several risk factors for the inspections to be initiated, including: potential pitfalls identified during the product registration process; adverse events; risks identified by testing agencies; whistle-blower tips; Marketing Authorization Holder violations; violations noted by other regulatory agencies; and repeat violations and other situations.
The agency will issue an inspection notice and holders of the product registration should then fill in and send back an inspection authorization and product information sheet within 20 work days. Master file and other required documents must be provided within 40 work days, and holders should cooperate fully and not delay, deter, avoid or refuse the inspections, the NMPA notes.
Manufacturers' sites aside, other relevant parties are also subject to the possible inspections including R&D partners, suppliers of active ingredients, excipients, and packaging materials, and the registration holder should coordinate and communicate with these.
All parties are expected to be consistent in following the manufacturing manual outlined in product registration files submitted to the NMPA, and to comply with China GMP standards.
During the inspections, manufacturers should maintain normal real-time production processes, open relevant areas to inspectors, and provide relevant files, records and digital data as required.
The agency may then issue one of three decisions - pass, pass with corrective actions, or fail - and can issue penalties for products that fail, ranging from meetings and warning letters to suspension of importation, import bans and product recalls, or in the worst case a sales ban and revocation of the Product Import Certificate. [Click here for the NMPA Overseas Inspection regulation]
Cooperation Key
The success of the expanded overseas inspections will hinge on cooperation from manufacturers and sufficient communication, noted the NMPA. Overall, the agency's grip over drug and device production compliance is tightening, noted Cui Hao, director of its Drug and Cosmetics Regulatory Bureau. The agency also said it will step up its own information sharing and disclosure of firm that do not cooperate with the inspections.
In such cases, the new regulation warns that companies will be issued with a failure notice.
From the editors of PharmAsia News
