FDA’s Rx Promo Citations Rise Slightly In 2016; Investigational Drug Promos Scrutinized
Executive Summary
Office of Prescription Drug Promotion seemed to expand its focus in issuing 11 letters last year.
For much of 2016, FDA seemed on pace to issue a record-low number of citations for Rx advertising, but in an end-of-year flurry, the agency issued six enforcement letters, bringing the tally to 11 – two more than the agency’s Office of Prescription Drug Promotion issued in 2015. That figure was a record low, which FDA retroactively matched for 2014 when it withdrew a letter to Pacira Pharmaceuticals Inc. regarding the promotion of Exparel (bupivacaine liposome injectable suspension).
The agency withdrew the Pacira letter in settling a lawsuit over FDA’s interpretation of Exparel labeling. (Also see "FDA Makes Broad Concessions In Settling Pacira Promotion Suit" - Pink Sheet, 15 Dec, 2015.)
The slight uptick in 2016 follows a steady decline since the agency’s 51 enforcement letters in 2010 (see chart below). The number has fallen even more sharply since 1998, when the office issued 157 warning and untitled letters.
Asked last year about the drop in citations, FDA said that reviewing the number of compliance actions that the agency takes within a year timeframe does not take into account the work that OPDP does on other priorities to assist companies with compliance, such as policy and guidance development and core launch reviews. “OPDP uses a risk-based approach to carefully allocate its resources among these activities to have the greatest beneficial health impact,” the agency said. (Also see "FDA's Rx Promotion Citation Drought Continued In 2015" - Pink Sheet, 4 Jan, 2016.)
However, the enforcement letters issued in 2016 suggest that FDA has been looking at a broader range of issues. Four companies were cited for promoting investigational drugs and two for TV ads that communicate serious risk information while distracting visuals appear on the screen.
Nikki Reeves, a partner at King & Spalding, said FDA is taking a more measured approach to enforcement actions and is moving away from citing off-label promotions and claims in the wake of successful First Amendment challenges to such actions.
Reeves noted that FDA also seems to be focusing on promotional material where it believes there is a minimization or omission of risk information. “It will be much more difficult for a manufacturer to successfully challenge FDA enforcement action taken on the basis of minimization of risk information,” she said.
‘Cautionary Tale’ In TV Ad Citations
The letters regarding direct-to-consumer TV ads, issued to Sanofi and Celgene Corp. on Dec. 12, are particularly notable. While FDA has previously criticized TV ads for obscuring risk information with audio and visual messages, these letters are unusual in focusing solely on this issue.
The Sanofi direct-to-consumer TV ad for Toujeo (insulin glargine) showed a man dancing to the song “Let’s Groove” as he was cooking, walking his dog, mowing his lawn, and picking tomatoes with his children. And Celgene’s ad for its plaque psoriasis drug Otezla (apremilast) showed people taking selfies at a park and dancing at a rooftop party. (Also see "DTC Trouble: ‘Attention-Grabbing Visuals’ Undermine Risk Info, FDA Tells Celgene, Sanofi" - Pink Sheet, 15 Dec, 2016.)
These letters are “a cautionary tale for companies looking to continue doing broadcast ads,” Reeves said.
OPDP Enforcement Letters
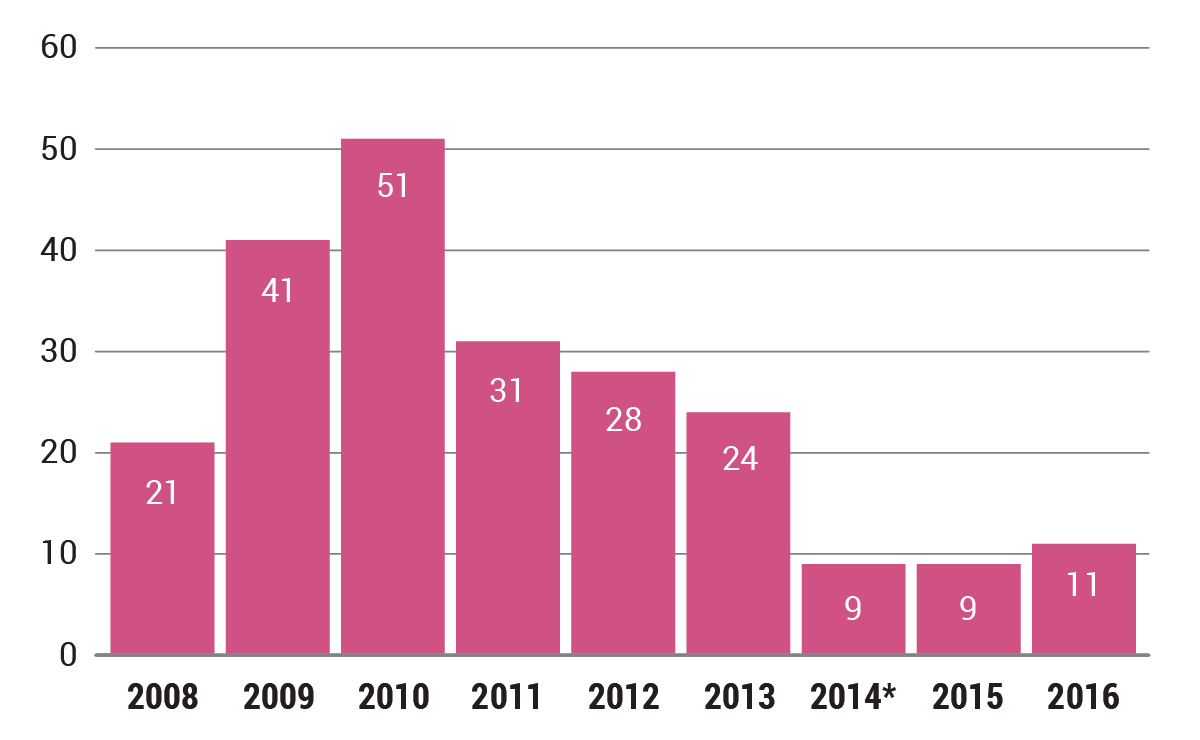
*FDA withdrew a 2014 letter to Pacira Pharmaceuticals
Focus On Investigational Drug Claims
FDA objections to promotions for investigational drugs also stands out. The last letters issued for the year, dated Dec. 21, went to Chiasma Inc. and Zydus Discovery DMCC for YouTube videos posted on their websites.
In its letter to Chiasma, FDA said a video in which experts discuss new advances in acromegaly suggests that Chiasma’s investigational new drug Mycapassa (octreotide) is safe and that its effectiveness has been proven in clinical trials.
The enforcement action comes after FDA issued a “complete response” letter for Mycapassa. In an April press release, Chiasma reported that FDA had told the company it did not believe the Mycapassa application had provided substantial evidence of efficacy to warrant approval of the drug and that Chiasma would have to conduct another clinical trial.
Mycapassa is an oral reformulation of Novartis AG’s Sandostatin, which originally had to be injected subcutaneously three times a day. Novartis later introduced a monthly intravenous formulation (Also see "Chiasma Looks Ahead To First Approval With "Revolutionary" Technology Opportunity" - Pink Sheet, 21 Mar, 2016.)
The letter to Zydus cites a video about the mechanism of action of its diabetes investigational drug Lipaglyn (saroglitazar). FDA says the claims and presentations in the video make conclusions that the drug is “indicated for the treatment of diabetic dyslipidemia and hypertriglyceridemia with Type 2 diabetes mellitus,” is “superior” to other molecules, and is not associated with many serious risks that are generally attributed to these other molecules with similar mechanisms of action.
FDA acknowledges that saroglitazar has been approved for use in another country – it was approved in India in 2013 – but it says the claims and presentations in the video, including the broad statements regarding the drug’s approval as the “world’s first,” suggest that the drug is approved throughout the world, including in the United States.
FDA sent another letter citing investigational drug promotions in September to Durect Corp. and Pain Therapeutics Inc. for their Remoxy (oxycodone controlled-release) websites suggesting the investigational drug is safe and effective. The agency cited “considerable public health concerns” about the promotions. (Also see "FDA’s Promotional Enforcement Still Playing It Safe" - Pink Sheet, 14 Nov, 2016.) Pain Therapeutics subsequently reported that it had received a complete response letter from FDA requiring new studies and data to support Remoxy abuse deterrent claims. (Also see "Remoxy's Woes Stoke Criticism Of FDA's Review Of Abuse-Deterrent Opioids" - Pink Sheet, 30 Sep, 2016.)
Celator Pharmaceuticals Inc. (now Jazz Pharmaceuticals PLC) was dinged for a placard at the American Society for Clinical Oncology annual meeting describing the company’s investigational high-risk acute myeloid leukemia candidate Vyxeos (cytarabine/daunorubicin) liposome injection. FDA said the placard and area surrounding the display did not indicate that the drug product is investigational. (Also see "FDA's Decline In Promotion Enforcement Highlighted By Celator Booth Letter" - Pink Sheet, 7 Sep, 2016.)
In recent years, the agency has rarely issued citations for promotional pieces about investigational drugs. In 2015, OPDP sent one such letter, to a researcher at the University of California at Los Angeles’ Semel Institute for Neuroscience & Human Behavior about a drug being investigated for use in brain PET scans to diagnose traumatic brain injuries, Alzheimer’s disease, and other neurological conditions. And in 2013 the agency sent a letter to CBA Research Inc. objecting to a website promoting an investigational cancer drug as safe and effective.
Webpage For Codeine Combo Lacks Risk Info
The other end-of-year letters, issued Dec. 13, were warning letters to Spriaso LLC and United-Guardian Inc.
In its letter to Spriaso, OPDP said the company’s webpage for Tuxarin ER (codeine phosphate/chlorpheniramine maleate) extended release tablets failed to communicate any risk information about the product and included claims that suggest it is safer than its competitors. The product labeling contains a boxed warning regrading respiratory depression and death, which have occurred in children who received codeine following tonsillectomy and/or adenoidectomy.
The letter also says the webpage does not disclose that the product is not indicated for pediatric patients under 18 years of age. And FDA says the company failed to submit the webpage for agency review at the time of initial dissemination, as required. OPDP requested that the company submit a plan of action to disseminate corrective messages to the audiences that received the violative promotional pieces.
Spriaso’s website now only states the ingredients in Tuxarin ER. It is the Salt Lake City, Utah-based company’s sole approved product. Spriaso has two other cough products in its pipeline, one is a combination of codeine and guaifenesin and the other is a combination of hydrocodone and guaifenesin.
Email Link To Prescribing Info Insufficient
FDA’s letter to United-Guardian objected to an email for its Renacidin (citric acid/glucono-delta-lactone/magnesium carbonate) irrigation solution for failing to include any risk information and making false or misleading benefit claims.
The product is indicated for dissolution of bladder calculi by local intermittent irrigation through a urethral catheter or cystostomy tube as an alternative or adjunct to surgical procedures. FDA noted that it is contraindicated in the presence of demonstrable urinary tract extravasion and that the product labeling contains warnings about fever, urinary tract infection, persistent flank pain, and sepsis, among other things.
FDA noted that the email includes a link to complete prescribing information but said this does not mitigate the omission of the risk information. OPDP also said the claims that Renacidin “simplifies long-term catheter care” and has “easy 30 mL dosing and delivery” are unsupported. It asked the company to provide a plan for disseminating corrective messages.
United-Guardian President & General Counsel Ken Globus said the email was sent to urologists once in June 2016 and that the ad included in the email also had been placed in two medical journals in April 2016, running one time in each journal. The email and ads were provided to FDA at the time they were released. Globus said the company has sent FDA a formal written response with suggestions as to how it can best address the agency’s concerns and will act after receiving FDA’s comments.
Patient Co-Pay Voucher Among 2016 Citations
Of the 11 letters OPDP issued during the year, three were warning letters and eight were untitled letters. In addition to the warning letters to Spriaso and United-Guardian, FDA also sent a warning letter to Shionogi Inc. for a false and misleading “Patient Co-Pay Assistance Voucher” for its head lice drug Ulesfia (benzyl alcohol) which omitted risk information.
Among the other letters of 2016, Hospira Inc., a Pfizer Inc. unit, was cited for a YouTube video about its sedative Precedex (dexmedetomidine hydrochloride), which OPDP said omitted risks and material facts. And Supernus Pharmaceuticals Inc. received a letter for a Spanish video for its seizure drug Oxtellar XR (oxcarbazepine) in which an expert suggested the drug is intended for unapproved uses. FDA said the violations were “concerning from a public health perspective” because they create a misleading impression about the drug’s safety and effectiveness and suggest a use for which the labeling does not provide adequate directions for its safe and effective use. (Also see "FDA’s Promotional Enforcement Still Playing It Safe" - Pink Sheet, 14 Nov, 2016.)
