Supplements Part Of The Past As Perrigo Frames Future Around Rejecting Mylan Tender
This article was originally published in The Tan Sheet
Executive Summary
The OTC private label leader says its supplement business will be the first asset it sells as it begins a streamlining process, which also includes supply chain consolidation and staff reductions, aimed at $35m in annual cost savings.
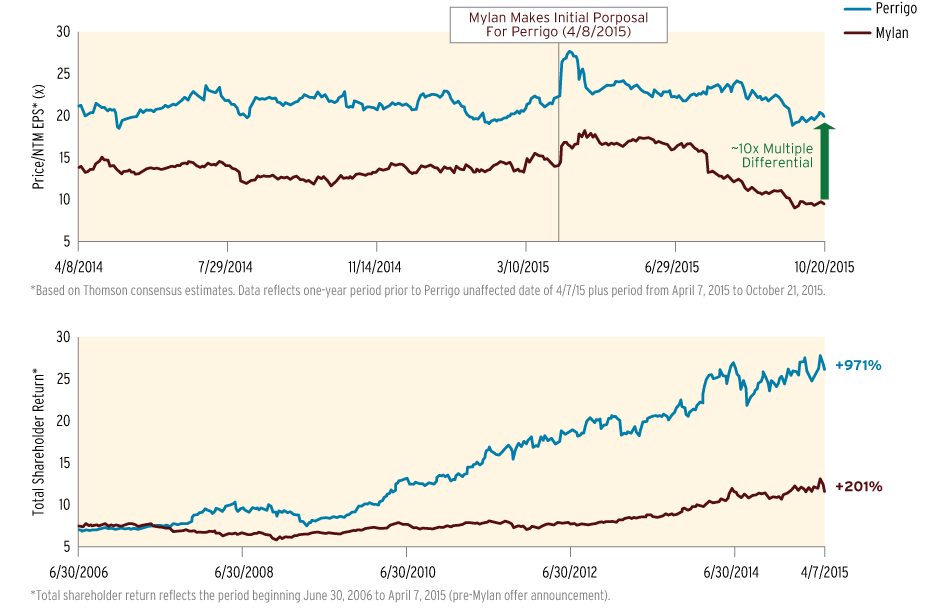
Perrigo Co. PLC puts its dietary supplement business on the block, pointing past a company record of $1.34bn net sales in its latest quarter, including $977m from consumer health products, to sway shareholders against Mylan NV’s hostile takeover bid.
The OTC private label giant on Oct. 22 also directly criticized Mylan’s tender during its calendar 2015 third-quarter earnings briefing for analysts. A presentation Perrigo made available includes numerous slides comparing its share value to Mylan’s, questioning Mylan’s reasons for making the tender and noting market analysts’ support for Perrigo remaining on its own.
The presentation also notes Perrigo board’s statements on Mylan’s tender of $75 cash and 2.3 shares for each Perrigo share. A slide features banners with statements including the tender “is a Bad Deal for Perrigo’s Shareholders” that “Substantially Undervalues Perrigo and Will Destroy Shareholder Value.”
Perrigo CEO Joe Papa quickly referenced the firm’s resistance to Mylan in his opening remarks. “Even with all the noise over the past six months,” he said the firm’s latest results “provide yet again evidence of … the strength of our global platform and the unrivaled position of our consumer health care business.”
“To keep it very simple, just by executing on our existing plans, Perrigo beats Mylan's numbers and Perrigo shareholders retain control of the company that has driven superior [share price/earnings] multiple year after year,” he added later.
Litigation To Halt Tender Process
Mylan, incorporated in the Netherlands while maintaining its administrative headquarters in the Pittsburgh area, planned when it announced it would make its offer directly to Perrigo shareholders that it would take over the firm only with 100% of shares tendered, but later said it would close the offer with a majority stake, making Perrigo a controlled subsidiary.
Mylan’s authority to operate Perrigo as majority owner is one of the many topics on which the firms disagree in dueling complaints filed in federal court New York. Perrigo contends Irish regulations would buffer it from Mylan essentially taking the helm but Mylan expects to immediately put Perrigo under its thumb.
Oral arguments were scheduled Oct. 21 on Perrigo’s complaint to halt the tender or to delay the process and require Mylan to make clarifying statements to Perrigo shareholders, and on the counter-complaint Mylan filed to dismiss its takeover target’s litigation (Also see "Perrigo Stays Course On Expansion Despite Mylan Litigation Storm" - Pink Sheet, 6 Oct, 2015.).
Mylan’s offer values Perrigo at $27.4bn based on its closing share price on Sept. 8 – the last trading day before the tender – and would give Perrigo shareholders a 40% stake in the combined company. The tender ends Nov. 13.
Irish securities rules allow firms that are tendered at least 80% of an acquisition target to force out remaining shareholders; if more than 50% but less than 80% are tendered, the rules require an additional tender for the remaining shares within six months. Ireland also mandates that with 50% or less of Perrigo shares tendered, Mylan’s offer is rejected and the shares are returned.
In addition to making its vitamin, mineral and supplement products business available, the Dublin-based firm said it will look at selling other assets and will eliminate redundant posts in its organizational structure, looking at reducing its workforce by around 800, or 6% of its current global headcount.
Perrigo’s changes to reduce costs and increase margins, aimed at convincing shareholders of a greater return than offered in Mylan’s tender, also include consolidating its global supply chain in Ireland and a $2bn share repurchase plan through 2018, including $500m in the current quarter, forecast at $9.30 per share.
The organizational changes include promoting John Hendrickson from executive vice president for global operations and supply chain to the firm’s new president post.
Its nutritionals business also includes infant formula products, which are generating stronger sales and will not be divested, according to the firm.
$302m Branded Product Sales
For its calendar 2015 third quarter, Perrigo reported record adjusted margins of 49% gross and 27% operating as its adjusted net income increased 38% to a record $258m and adjusted diluted earnings per share increased 26% to $1.76. Organic sales growth of 10%, on a constant currency basis, to $95m drove overall net sales growth of 41%, highlighted by organic net sales of $95m.
While US OTC private label sales continue to comprise the bulk of Perrigo’s consumer health business, the firm reported $302m net sales from branded products, including new product sales of $31m, as it integrates the operations of European firms and brands it has acquired over the past year. The firm said branded-consumer adjusted operating income during the July-September period was $44m, 15% of sales.
Overall consumer product net sales grew 8% on a constant currency basis to $675m, including new product sales of $65m and a $49m increase in sales of existing products, primarily in the gastrointestinal, infant formula and cough/cold categories.
Perrigo, which also markets specialty Rx topicals and pet care products, noted its consumer business increases were offset by loss of $52m sales from discontinued products, a $27m decline in existing products, primarily in animal health, diabetes and analgesics, and a $14m impact from foreign currency exchange. The segment’s adjusted operating income grew 48% to $139m due to higher gross profit contribution and relatively lower operating expenses compared to the year-ago quarter.
The firm entered the branded consumer product space with its acquisitions of German firms Omega Pharma NV, which markets OTCs and nutritionals, and Naturwohl Pharma GmbH, maker of meal- replacement and weight-loss supplement drink mixes, and 11 international product lines from GlaxoSmithKline PLC and Novartis AG. It more recently announced an exclusive licensing of French firm Flamel Technologies SA’s LiquiTime technology to develop a portfolio of OTC extended-release suspension products for marketing in the US and other products (Also see "Perrigo Stays Course On Expansion Despite Mylan Litigation Storm" - Pink Sheet, 6 Oct, 2015.).
Divestment Starts With VMS
Papa in June said Perrigo was focused on increasing sales of its gummy vitamins to drive growth of its VMS business, which was not taking market share from major firms operating only in the nutritional products space. He said the firm would consider divesting the unit unless it generated higher return on investment capital (Also see "Perrigo Considers Divesting Supplement Business, Remains Mylan-Averse" - Pink Sheet, 8 Jun, 2015.).
The US “VMS business delivers excellent products to our long-standing customers,” the CEO said during the earnings briefing, but “we think that it will thrive more fully under new ownership.”
Although net VMS sales during the quarter grew 5% on product launches, “its divestiture will enhance our revenue and operating margins earnings and ROIC,” Papa said.
The firm entered the gummy vitamin and supplement market through an agreement announced in January with Ferrara Candy Co. Inc. to manufacture the products Perrigo would provide to US retailers (Also see "Perrigo Fills “Moat” To Protect Growth With Gummy Vitamins, European Flavor" - Pink Sheet, 13 Jan, 2015.).
Perrigo forecasts $35 million in annual cost savings from its streamlining, beginning with divesting the VMS business.
“VMS has been a business that we've talked about possibly looking at strategic alternatives for some time,” said Chief Financial Officer Judy Brown.
Brown said “other portfolio refinements” also factor in the costs savings estimate. “We're specifically referencing VMS today because we are going to kick that process off soon,” she said.
Perrigo Compares Share Value To Mylan
Perrigo Co. PLC
