Surge In Applications Triggers User Fee Inflation
Executive Summary
With full NDA and BLA submissions to FDA now growing faster than INDs and supplements, user fees are increasing more rapidly as well.
In addition to being burdened with more meetings and work during most new drug reviews, FDA staff also is seeing more applications, which is helping increase user fees.
The average number of NDAs and BLAs the agency reviewed increased substantially between 2010 and 2015, according to the workload adjustment used to calculate FY 2016 prescription drug user fees.
The proportion of the total human drug review workload they comprised also increased, FDA said in an Aug. 3 Federal Register notice announcing the new fees. Now NDAs and BLAs account for nearly the same amount of work as commercial INDs, which the last few years had taken most of FDA’s time.
The result was a workload adjustment of more than 11%, which increased the overall amount of revenue the PDUFA program would be expected to generate in the coming fiscal year.
It also coincided with an increase in the application and other fees that are part of the program. However, the increases were muted compared to previous years in part because of an increase in the number of fee-paying full application equivalents (Also see "PDUFA Fees To See Small Rate Increase In 2016" - Pink Sheet, 31 Jul, 2015.).
FDA used a three-year average of the number of NDAs and BLAs that it received to help determine the workload adjustment.
During the most recent three-year period, which ended June 30, 2015, the agency averaged 148.3 NDAs and BLAs received combined. That was more than 19% higher than the baseline three-year period, which ended June 30, 2012 (see chart).
Average NDA/BLA Receipts
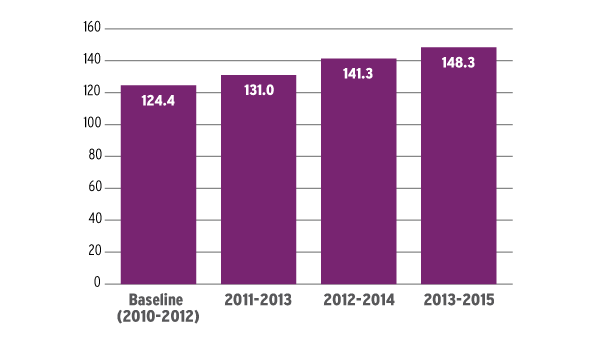
Recent three-year averages of the number of NDAs and BLAs FDA has received are increasing, which affects the prescription drug user fee program workload adjustment.
Source: Federal Register notices
The increase in applications has been accompanied by an increase in approvals in recent years.
The Center for Drug Evaluation and Research cleared 41 new molecular entities and novel therapeutic biologics during calendar year 2014, the most since 1996. The center approved 39 in 2012 and now has approved more than 25 NMEs and novel biologics in four consecutive years (Also see "FDA Solidifies 2014 As Breakthrough Year With December Drug Approval Rush" - Pink Sheet, 5 Jan, 2015.).
PDUFA V brought with it an extension of the application review time, but also more sponsor interaction mid-review as part of an effort to promote more first-cycle approvals.
An interim assessment of what is known as “the Program” was mostly positive, although some within FDA were concerned about the increase in work associated with it (Also see "PDUFA V Reviews Are ‘Best Ever’ For Some Sponsors, Maybe Not FDA" - Pink Sheet, 6 Apr, 2015.).
NDAs And BLAs Carry More Weight
The average number of active commercial INDs also has increased significantly since the baseline period, from an average of 6,830 during the 2010-2012 period to 7,375.3 in the 2013-2015 period.
FDA said in the notice that NDAs and BLAs were about 38.9% of its human drug review workload. Active commercial INDs were 39.2% of the workload, while efficacy supplements were 6% and manufacturing supplements were 16%, according to the notice.
During previous years, the averages have showed INDs constituted the bulk of the work. They were more than 41% of the workload during the previous two three-year periods.
The efficacy supplement workload steadily decreased during the same time, while the manufacturing supplement workload increased (see table).
|
PDUFA Workload Weighting Factors |
||||
|
Type |
2011-2013 Average |
2012-2014 Average |
2013-2015 Average |
2011-2015 Change |
|
NDA/BLA |
38.6 |
37.3 |
38.9 |
.78% |
|
Active Commercial INDs |
41.4 |
41.4 |
39.2 |
-5.31% |
|
Efficacy Supplements |
9.3 |
7.5 |
6.0 |
-35.48% |
|
Manufacturing Supplements |
10.7 |
13.8 |
16.0 |
49.53% |
|
Source: Federal Register notices |
||||
Manufacturing issues have gained more attention during PDUFA V in part because of the breakthrough therapies designation.
The program’s increased and earlier interactions between FDA and sponsors in the hopes of bringing a potentially significant treatment advance to market faster have forced manufacturing operations to be prepared faster (Also see "‘Breakthrough’ Drugs Get ‘Road-MaPP’ – Complete With Communication Timetable" - Pink Sheet, 29 Jul, 2014.).
FDA also has faced criticism in recent years for its facility inspections creating drug shortages. House members said the agency was not being flexible in requiring manufacturers to make changes following an inspection that in some cases forced them to shut down in the interim (Also see "Drug Shortages Mostly FDA’s Fault, House Oversight Committee Concludes" - Pink Sheet, 15 Jun, 2012.).
Drug Review Costs Recover From Sequester
FDA also managed to boost its spending on human drug review in FY 2014 after the budget sequester forced a dramatic decrease.
The agency pushed total costs to $1.08bn in fiscal year 2014, the highest in at least six years. It was an 11.5% increase from FY 2013 when the agency spent $966.2m.
FDA said the sequester forced reductions in travel and hiring that limited its spending on human drug reviews in FY 2013 (Also see "Thanks Sequester: FDA’s Human Drug Review Costs Decrease To Pre-FY 2011 Level" - Pink Sheet, 11 Aug, 2014.).
Much of the increase in FY 2014 came among non-personnel, compensation and benefit costs, such as rent, travel and transportation. The amount jumped nearly 24% to $492m from $398m the previous fiscal year.
The 2014 non-personnel cost was the highest in the last six years.
FDA’s rent costs increased significantly after it opened the new four-building Life Sciences-Biodefense Laboratory at its White Oak headquarters. It allowed the agency to move employees out of leased space, but the rent the agency pays to the General Services Administration was more than it had been charged in the previous facilities (Also see "FDA’s Rent Is Too Damn High: Consolidated Labs Cost More Than Off-Campus Space" - Pink Sheet, 9 Feb, 2015.).
Personnel costs also increased 3% to $585.3m in FY 2014. But the amount was still less than was spent in FY 2011 and FY 2012.
Personnel also constituted 54% of the total human drug review cost. That is in contrast with the generic drug review program, where non-personnel costs were the majority of the total spent (see related story, (Also see "Generic Drug Review Costs Still Largely Non-Personnel Related" - Pink Sheet, 10 Aug, 2015.)).
FDA did not request many new full-time equivalent employees for the human drugs program in its FY 2016 budget request, which may mean the employee count will remain stable for the near future (Also see "FDA Personnel Growth Looks To Have Plateaued For Drugs Program" - Pink Sheet, 16 Feb, 2015.).
