To Congress, From Pharma: Love, Hate And Fresh Ideas For 21st Century Cures
Executive Summary
Industry and key stakeholders weigh in on House Commerce Committee’s first take at biomedical reform; brand companies offer lots of positive feedback but want many modifications; generic companies criticize multitude of new exclusivity provisions for brands.
A review of drug industry comments on the first version House Energy and Commerce Committee’s 21st Century Cures Initiative legislation reveals a bill that is being largely embraced by branded product sponsors, and criticized by generic companies.
Branded companies do have their fair share of tweaks and modifications though, and a few provisions they’d rather not see move forward – expanded access provisions top that list. However, branded company suggested revisions generally come on top of initial support for the starting draft measures.
Generic makers who commented on the discussion document largely spent their time criticizing the many new exclusivity provisions offered in the bill, which they fear will upset the balance between incentivizing innovation and availability of affordable generics created by the Hatch-Waxman Act (Also see "Antibiotics Top List Of New Exclusivity Benefits In Draft Cures Legislation" - Pink Sheet, 27 Jan, 2015.).
One area of agreement for both brands and generics was in modernizing and reforming clinical trials. The Generic Pharmaceutical Association said that “by accelerating the development phase of the prescription drug cycle, we can spur new cures for patients and our manufacturers can sooner bring cost-saving generic versions on the market.”
And despite the nearly 400-page discussion document, companies still have dozens of new legislative proposals they want lawmakers to consider (Also see "Pharma’s Cures: Breaking Down The House’s Biomedical Reform Bill" - Pink Sheet, 2 Feb, 2015.). For branded companies, payment reform came up frequently as an issue they would like addressed in the legislation.
“The Pink Sheet” reviewed all biopharmaceutical company comments on the draft posted by the Energy and Commerce Committee, along with comments from key trade organizations and lobbying groups to get a sense of what’s popular and what’s not in the first itineration of the House’s biomedical reform effort.
Nearly 140 organizations submitted comments to the Energy and Commerce Committee. Notably, the bulk were from disease advocacy organizations and physician groups, not industry. This review focused on industry and key stakeholders. It also includes some comments from organizations who spoke directly to “The Pink Sheet.”
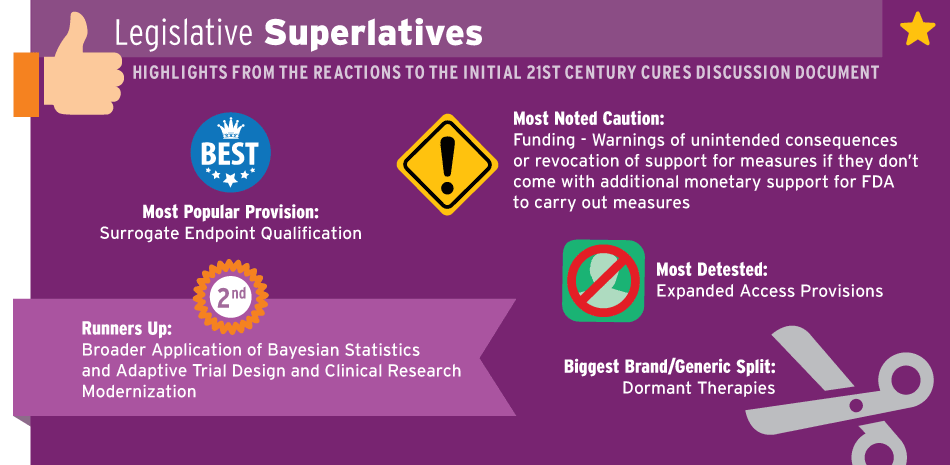
We deciphered sentiment on the measures included in the bill and listed additional provisions the industry is calling for. It’s important to remember most organizations didn’t comment on every element of the bill – while it’s likely they wouldn’t want to hide any criticism, it’s possible they liked sections they choose not to highlight – for example many branded companies didn’t specifically draw attention to some of the new exclusivity provisions though they likely aren’t opposed to them. Our superlatives graphic and following chart help pull out what issues are drawing the most attention in the comments, for better or worse.
|
General Comments On Lack Of New Funding |
|
|
Criticism |
Amgen wants drafters to “be mindful of the inherent tension that exists between new requirements placed on the agency and how they could impact the current FDA review model by re-directing agency resources. While individual provisions may clearly improve the system, ultimately, the legislation will have to be weighed by the sum of its parts in terms of the impact on the agency in its continued ability to focus on reviewing new treatments for patients.” Association of Clinical Research Organizations (ACRO) recommends that “to the extent the FDA would be given additional responsibilities, we urge Congress to simultaneously provide additional appropriations for the FDA to carry out an expanded mission. We believe the current ratio of appropriations to user fees should be maintained in the interest of sound public policy and regulatory independence.” National Organization for Rare Disorders (NORD) notes that “while we are encouraged by many of the provisions contained within the discussion draft, we cannot support the discussion draft in its current form” in part because many provisions are too burdensome and prescriptive on FDA with little to no additional resource. Faster Cures says “new statutory responsibilities outlined in the draft will need to be accompanied by new resources so that these proposals do not divest scare resources from existing responsibilities.” |
|
New Proposals Offered By Commenters |
|
|
Vivus |
Authorize coverage of obesity medication under Medicare Part D |
|
NORD |
FDA Office of Patient Relations should be elevated to the level of the FDA commissioner's office. “Without this office, the patient engagement initiatives contained within this discussion draft will only strain the limited resources the FDA has for patient engagement even further.” Pediatric Rare Disease Priority Review Voucher Program should be made permanent. Orphan Products Board – wants requirement that the currently dormant (but statutorily mandated) board report to Congress on its activities. Patients’ Access to Treatment Act or similar measure should be included to ensure patients can access therapies and are not stopped by prohibitive co-insurance levels within specialty tiers. Off-label coverage for therapies in Medicare Part D even if there are no published, reviewed studies on use. Conflict of interest rules adjusted for patient advocates serving as FDA special government employees on drug reviews. |
|
GPhA |
Prohibit abuse of risk evaluation and mitigation strategies (REMS) as a means to delay generic entry. |
|
Eli Lilly |
Improve reimbursement of medicines under recommendations included in the Biotechnology Industry Organization’s delivery-side proposal submitted to the committee in December. Lilly worked with BIO to develop the proposals, including:
|
|
Mylan |
Create incentives for companies to help prevent or alleviate a drug shortage of a medically necessary product.
Encourage earlier patient access to generic medicine and foster competition, ensuring approval on the earliest legally eligible date.
Expedite and encourage investment in the development of complex generic drugs. Increase FDA transparency and help identify areas for administrative improvement through reports to Congress on measures related to ANDAs and inspections such as number of inspections conducted by country, number of ANDAs FDA was not able to approve by day 181 and delays in ANDA approvals. |
|
Genentech |
Create “pass-through” process for two to three years to allow separate payment for certain new drugs and biologics, similar to pass-through payment in hospital outpatient setting. Wants clinical trial coverage strengthened so that prevention, detection and treatment of complications arising from clinical trials are covered by group health plans and insurance issuers as routine patient costs. Calls for corrections to methodologies that measure cost and quality to better support personalized patient-centered care so patients can access the most clinically appropriate treatment and physicians are not penalized. Also wants budgetary guardrails against decisions that are primarily based on short-term financial gains at the expense of long-term health. |
|
Novartis |
Allow Medicare Part D beneficiaries to appeal for a lower-tier cost-sharing amount when they have a medication placed on a specialty tier. |
|
Patient-Focused Drug Development (Title I, Subtitle A) |
|
|
Reaction |
Pro — Alzheimer’s Association, Alzheimer’s Foundation of America, ACRO, Sarepta, Genentech, BIO So-So — Novartis, Eil Lilly, GlaxoSmithKline, NORD, Faster Cures |
|
Compliments |
BIO said patient-focused drug development is one of their two big priorities for 21st Century Cures.
Genentech agreed with need for additional guidance documents on patient reported outcomes. Strategies for determining disease and treatment burden, function and use of registries are important.
NORD strongly supports efforts to strengthen patient’s voice within FDA drug review process (but does not like how current language of provision is written). |
|
Criticism |
Novartis worries biopharma industry is excluded from workshop involving multiple stake-holders. Eli Lilly also said pharmaceutical industry should be participant in relevant discussions on this topic. NORD is concerned that the provisions may create an undue burden on FDA while adding only limited value to patients. Faster Cures says language should “be strengthened in order to provide greater regulatory certainty about the collection, applications and integration of information about patient experiences, expectations and trade-offs.” |
|
Changes Requested |
Genentech suggests process through which sponsors may approach FDA for discussion about use of PRO data as labeling-enabling endpoints could similar to that for biomarkers (Title I, Subtitle B). Report to congress may not be necessary and preferred to have focus on guidance documents.
Lilly wants guidance on methodologies to incorporate patient perspective into drug development to be based on input from the social sciences. Legislation should explicitly state that patient data collected in scientifically valid methods should be considered in regulatory decision making and should transparently be reflected in reviewers’ benefit/risk assessment frameworks.
GSK thinks proposal could be “significantly improved” if data generated by biopharmaceutical companies is included in the definition of patient experience data. Biopharma companies should also be included as required representatives for workshops. |
|
Surrogate Endpoint Qualification (Title I, Subtitle B) |
|
|
Reactions |
Pro — Alzheimer’s Association, Alzheimer’s Foundation of America, ACRO So-So — AstraZeneca, Genentech, Novartis, Eli Lilly, GSK |
|
Compliments |
Genentech supports timelines proposed for qualification of surrogates, likes idea for public forums, as well as publicly disclosing information on surrogate endpoint qualification applications received, reviewed and decided on. |
|
Criticism |
Industry concern that scope is too narrow. |
|
Changes Requested |
AstraZeneca wants scope expanded beyond surrogate endpoints to include all biomarkers, as validated biomarkers can be used in a wide range of other important applications to accelerate product development timeline and assure safer medicines.
Genentech discourages Congress from requiring FDA to do formal reporting and instead adapt the measures to passively monitor program performance.
Novartis wants patient-reported outcomes and other drug development tools included in the process.
Lilly says public-private partnerships should be established to help operate biomarker qualification processes. FDA would need to be part of these partnerships in order for them to be successful and FDA should remain the final decision-maker. |
|
Approval Of Breakthrough Therapies (Title I, Subtitle C) |
|
|
Reactions |
Pro — GSK Con — ACRO So-So—Genentech |
|
Compliments |
GSK supports giving FDA increased flexibility by enabling the possibility to approve breakthrough drugs on the basis of early stage safety and efficacy data (Phase II). Also supports the language that allows post-market requirements to be fulfilled using data sources other than randomized clinical trials, including from observational studies and registries. |
|
Criticism |
ACRO worries use of “early stage clinical data” may be insufficient to make decisions to approve new drug, pointing out that many compounds fail in Phase III, meaning approving a new drug with only Phase II data “presents considerable risk.” Also questions whether Phase II trials will study enough patients for approval (excluding rare diseases) even with post-market assessment. “In order to preserve credibility and independence, the FDA must maintain the flexibility to determine the appropriate amount of clinical data needed to make an approval decision,” ACRO says. |
|
Changes Requested |
GSK wants draft language amended so that supporting evidence for breakthrough products isn’t limited to what is described in legislation.
Genentech: “We need more information about what problems this section is addressing.” |
|
Antibiotic Drug Development (Title I, Subtitle D) |
|
|
Reactions |
Pro — ACRO, Genentech, Infectious Disease Society of America (IDSA), Pew Con — GPhA So-So — Novartis |
|
Compliments |
ACRO specifically references its support for use of adaptive trial designs.
Novartis supports keeping the limited population pathway to antibiotics and antifungals only. “Further expansion could be a slippery slope for FDA to impose its authority into the practice of medicine where a product, once on the open market, is restricted in its use but not for reasons of safety or efficacy.”
IDSA strongly supports provisions that help guide appropriate use, including pre-review of marketing materials and monitoring the use of drugs approved under the limited population antibiotic drug approval (LPAD) pathway, as well as monitoring patterns of resistance. Likes option for companies to be able to transfer some of their exclusivity for qualified antibiotics to other products/companies. |
|
Criticism |
GPhA is concerned about the “wildcard,” or transferable, exclusivity that would be given to GAIN Act products. “It is premature to add additional exclusivity on top of the GAIN Act after only two years.” Says proposal could have “unintended consequences.” |
|
Changes Requested |
Novartis worries about the requirement that a recipient of transferred exclusivity donate 5% of the product’s sales to NIH. That provision must be carefully framed, the firm suggests, so it does not become bad precedent or cause an unpredictable funding stream for NIH. Also requests one-year transferable wildcard exclusivity to sponsors of neonatal products.
Genentech wants exclusivity extensions to apply to biologics. Also notes report that would be required under legislation might be duplicative of other government reports on antibiotics. Genentech also said provision ensuring adequate hospital reimbursement of new antimicrobials is important toward encouraging development and that this provision should not be budget neutral; additional payments should not come from existing hospital inpatient payments.
IDSA says FDA should have flexibility on timeframes for meetings with LPAD sponsors so it could respond to unpredictable emergencies like Ebola outbreak. Also wants a prominent visual element on drug labels to make it easy for health professionals to quickly recognize the drugs are approved for a limited population and must be used prudently.
Pew also wants visual element or other branding for limited population products and for legislation to be less prescriptive on timelines to make pathway easier for FDA and sponsors. |
|
Expanded Access (Title I, Subtitle G) |
|
|
Reactions |
Pro — Alzheimer’s Foundation of America, NORD, IDSA Con — Novartis, GSK, BIO So/So — Amgen, Genentech |
|
Compliments |
Amgen supports expanded access task force if it would help to simplify and clarify the process, including looking at existing FDA regulations and guidance. Support finalizing FDA draft guidance and Q&A as written. |
|
Criticism |
BIO does not want expanded access requirement being legislated. Amgen wants manufactures to be able to preserve their judgment on providing drugs to a particular patient, worries about unintended consequences of patients being unwilling to join or continue with clinical trials in favor of getting drug through expanded access. Current proposal could “have an adverse impact on registration studies and slow progress towards product approval and also impair a company’s ability to deliver on post-marketing commitments.” Detail required in explanation for denying expanded access requests could cause debate and open manufacturer judgment to be second-guessed by others, Amgen said, noting some valid reasons for not providing drug could include confidential commercial information. With respect to GAO report, unclear how GAO would gain access to data on number of expanded access requests denied by sponsors and would place additional burden on sponsors to track and report the information that GAO would need to support its reports. Novartis opposes mandate linking development or posting of expanded access policies to regulatory designation mechanisms. Any requirement regarding expanded access must not deter eligible patients from enrolling in clinical trials. GSK does not support statutory mandate related to expanded access policies being made public and linked to regulatory designations. Instead said supports voluntarily posting general expanded access policies where they exist. |
|
Changes Requested |
Amgen would rather have expanded access policies on each company's own websites rather than given to HHS and displayed on single site. |
|
Communication Of Scientific And Medical Developments (Title I, Subtitle H) |
|
|
Changes Requested (Section in discussion draft was a placeholder and did not include any legislative language) |
AstraZeneca recommends Congress clarify the scope of permissible communications with payers prior to the launch of a new medicine. Amgen proposes statutory clarifications to aid dissemination of health care economic information to payers, formulary committees or other entities. FDAMA (1997 user fee law) permits manufacturers to disseminate health care economic information that is directly related to an approved indication to payers, formulary committees or other similar entities so long as it is based on “competent” and “reliable scientific evidence,” but manufacturers have been stifled from disseminating important information about value of their products because key terms are undefined and FDA has yet to issue guidance interpreting these terms. Genentech encourages Congress to explore opportunities to leverage work underway through Harvard Multi-regional Clinical Trial Center and TransCelerate BioPharma. GSK also pushes for pharma companies to have more freedom to communicate research findings, seeking regulatory guidance to define uniform methodology standards for comparative effectiveness and real world research that can be appropriately shared. |
|
Social Media Regulations (Title I, Subtitle I) |
|
|
Compliments |
Genentech says language would allow for significant broadening of information that can be communicated by social media. Supports timelines proposed for drafting and finalizing regulation and guidance. |
|
Changes Requested |
ACRO recommends FDA provide guidance around acceptable use of social media for clinical trial recruitment. |
|
Streamlined Data Review (Title I, Subtitle J) |
|
|
Reactions |
Pro — Genentech, Novartis Con — ACRO |
|
Criticism |
ACRO concerned that data summaries may be inadequate for adding indications in many cases. “FDA must maintain the flexibility and authority to establish appropriate data parameters for adding new indications.” |
|
Changes Requested |
Amgen wants to remove the presumption that full data sets should be submitted to FDA, saying current language implies that the default requirement is full data sets unless otherwise specified. “If a summary review is being done then the full data sets should not be required,” Amgen said. Genentech urges workshops on guidance documents or reports to Congress from FDA. Also believes FDA may be able to implement change with an internal CDER MAPP, which might be less burdensome to produce and clearer than a guidance document. Also proposed that a transparent process for additional indications be defined, rather than creating a list of expanded indications. |
|
Dormant Therapies (Title I, Subtitle L) |
|
|
Reaction |
Pro — ACRO, Eli Lilly, GSK Con — Mylan, NORD, GPhA |
|
Criticism |
NORD “cannot support a patent protection period of 15 years … as this is far too long a protection period, especially compared to other exclusivity periods.” GPhA worries “expansive definition of dormant therapies would sweep in drugs that would have been developed even without the special incentives or that have only marginal improvements over currently marketed drugs.” Section would create “a host of questions regarding generic drug and biosimilar applications including the effect of first applicants’ right to the 180-day exclusivity period. … It also creates questions around potential patent term extensions for changes made during the extended exclusivity period blocking generic entry in perpetuity.” |
|
Extended Exclusivity For Certain NDAs and ANDAs (Title I, Subtitle M) |
|
|
Criticism |
Apotex worries that existing three-year “new clinical studies” exclusivity could be extended by up to two additional years under certain circumstances. Apotex said this would “jeopardize the 180-day exclusivity rights earned by sponsors of ANDAs that qualify as “first applicants.” Lengthening “new clinical studies” exclusivity from three to five years will diminish the value of 180-day exclusivity as ANDA first applicants could find themselves forfeiting earned 180-day exclusivity before having a chance to use it. Further the criteria for qualifying for the extended 5-year exclusivity period such as greater patient adherence, reduced public health risks, reduced side effects and others “will be open to interpretation.” “This will saddle FDA with a difficult task,” and will likely lead to litigation by both innovator and generic drug sponsors that are “disadvantaged by FDA decision-making” regarding this provision. “Such litigation will divert scarce FDA resources that could be better spent fulfilling FDA’s public health mission.” Apotex also said 180 days for FDA to implement final regulations is not a realistic timeframe. Mylan is “concerned that some of the proposed exclusivity incentives may have unintended consequences.” Section “appears to eviscerate the careful distinction that has been made for years between the level of innovation and expenditure needed for developing a new chemical entity… and improvements made to existing drugs.” Provision could result in “evergreening,” where brand companies maintain a continual monopoly by strategically making relatively small incremental changes over time, Mylan worries. |
|
Changes Requested |
Apotex proposes revising three-year exclusivity provisions to mimic the operation of current 5-year “new chemical entity” exclusivity provisions to facilitate the submission of higher quality applications that do not force FDA to expend scarce resources on hastily prepared submissions. Proposal would increase new clinical studies exclusivity to five and one-half years. |
|
Orphan Product Exclusivity Extensions (Title I, Subtitle N) |
|
|
Compliments |
NORD said proposal would create an incentive for placing rare disease conditions on the label, thus increasing the likelihood insurance would cover the therapy for the rare condition. |
|
Changes Requested |
Genentech supports additional incentives in concept, but wants to work with committee on what would be most meaningful to encourage additional rare disease drug development. |
|
21st Century Cures Consortium (Title II, Subtitle A) |
|
|
Changes Requested |
IDSA wants grants provided for partnerships instead of individual non-profits or small businesses. This would make this initiative unique and fill void in the U.S. Also wants consortium to address diagnostics. Instead of terminating consortium in 2021, have an assessment in 2026. Thinks consortium needs similar funding to Innovative Medicines Initiative in Europe, which has a €3.3 billion budget over 10 years, for consortium to have significant impact. |
|
Medical Innovation Advisory Council (Title II, Subtitle B) |
|
|
Changes Requested |
“Although Genentech supports FDA and CMS – among other stakeholders – working together to promote and accelerate the development and delivery of innovative therapies to patients, we ask that the committee recognize that the unique missions of these two agencies remain distinct and not be commingled or compromised.” “CMS should not attempt to use its limited resources” to duplicate FDA’s mandate to for approval standards, Genentech says. Also requests modifications to make clear that decisions about coverage, coding and reimbursement should occur separate and apart from consortium and commission. |
|
Genetically Targeted Platform Technologies For Rare Diseases (Title II, Subtitle D) |
|
|
Changes Requested |
Sarepta wants clarification that extrapolation of data should only be allowable where that data is owned by the applicant or there is otherwise right of reference. |
|
Technology Which Advances Regulatory Efficiency (Title II, Subtitle E) |
|
|
Changes Requested |
GSK says clinical decision support (CDS) tools should not be regulated by FDA like medical software but should have some oversight to ensure it is derived from clinical practice, based on clinical evidence and customized for an individual patient. Commends committee’s efforts to develop guidelines and distinctions between software that should be regulated by FDA and software that requires less oversight, though would like the delineation between the two to be clearer. “Use of digital applications for continuous remote measurement of patients to better understand disease states and their response to treatment in a real world setting and in real time has the potential to transform and accelerate the way our industry discovers, develops and delivers new medicines and cures for patients.” Genentech requests clarity on the use of medical software in drug development. |
|
Clinical Trial Data Sharing (Title II, Subtitle F) |
|
|
Reactions |
Pro — Alzheimer’s Association, ACRO, Genentech, GSK, IDSA, Pew So-So — Lilly |
|
Changes Requested |
Genentech says claims data availability should not be limited to Medicare Parts A and B. Claims data for Medicare Advantage, Part D, and Medicaid should be made widely available as well. Believes data from clinical registries could be utilized as virtual controls for future clinical trials. Wants allocation of more funds and resources to develop such methods.
Supports efforts to maximize use of real-world data to inform and improve patient care but wants recognition that clinical guidelines are based on average patient and are not appropriate in every instance. Integrated practice guidelines should not be used to determine coverage and payment, Genentech says.
Lilly “supports the need to make available Medicare data for evaluation of new care models, quality improvement activities, and other patient care activities; however this will only be impactful if the data and the information generated from the data can be re-disclosed and communicated by all stakeholders in the healthcare system.”
Research using Medicare claims data produced by qualified researchers should be allowed to be published in peer reviewed journals and shared with the medical and scientific community, Lilly says. Prohibition of data use for marketing purposes precludes the use of analysis or data from being used even if it meets all of FDAMA 114.
GSK suggests committee consider provisions for access to both Sentinel System and PCORNet by industry and others. Wants clarification on whether the established clinical trial data sharing entity would conduct analysis and to which the dissemination of results would apply. |
|
Utilizing Real-World Evidence (Title II, Subtitle G) |
|
|
Reactions |
Pro — AstraZeneca, Genentech, Lilly, GSK, Pew So-So — ACRO |
|
Compliments |
Lilly supports enabling FDA to consider big data and real world evidence when establishing the safety and effectiveness of new medicines and line extensions as well as fulfilling post-approval commitments. |
|
Criticism |
ACRO: “Within limits there are tremendous opportunities for the use of real world evidence. … FDA must retain the authority to determine, when and to what extent, real world evidence presents a sufficient basis to make regulatory determinations.” |
|
Changes Requested |
AstraZeneca wants specific reference to Patient Centered Outcomes Research Institutes (PCORI)’s methodology report, which has been fully vetted and could serve as a basis for standards associated with patient centeredness, data registries and data networks.
Genentech urges more resources be placed towards new methods development.
Lilly requests explicit statement that real-world evidence may be used, in part, to demonstrate substantial evidence for regulatory decision-making purposes. Consortia may be needed to facilitate data interoperability and access, as well as methods development. |
|
Coverage With Evidence Development (Title II, Subtitle H) |
|
|
Reactions |
Pro — Alzheimer’s Association, ACRO Cons — Amgen, Genentech So-So — Alzheimer’s Foundation |
|
Criticism |
Amgen said provision should not apply to drugs and biologics. “Codifying CED for drugs and biologics is not necessary as the current statue already supports coverage of drugs and biologicals for their medically accepted indications.” Recommends provision be revised to focus only on devices and that it be done outside of the CED framework to avoid confusion with CMS guidance on CED.
Genentech wants explicit language that “CED is not appropriate for FDA-approved and medically accepted uses of drugs and biologics – that is off-label uses of drugs and biologics supported in certain compendia and peer-reviewed journals.” |
|
Changes Requested |
Alzheimer’s Foundation of America has several concerns with current CED it wants addressed in Cures legislation, including very limited coverage during the CED period, and no coverage while trial data are being analyzed. |
|
Combination Products (Title II, Subtitle I) |
|
|
Changes Requested |
Amgen offers own proposal, previously submitted to Congress, on improving combination product regulation. In modifications to the existing proposal, Amgen says FDA Office of Combination Products should not act as the coordinator for communications during premarket review because Office is not staffed to do this; instead this should be the role of the agency center that is primary reviewer of the application. Amgen also wants requirement that guidance, regulations and policies for combination products be followed regardless of whether the FDA center is the “lead” center on the combination product or the consulting center. Genentech suggests using lessons learned from resolving challenges in regulation of in vitro diagnostic products for targeted therapies to guide changes with respect to regulation of combination products. Says GAO report as called for in draft document might be useful but that resources might be better spent by FDA on the development of internal MAPPs (manual of policies and procedures) and guidance on handling combination products. Lilly questions effectiveness of adding GAO report because it may produce counter-productive recommendations. |
|
Modernizing Regulation Of Diagnostics (Title II, Subtitle J) |
|
|
Changes Requested (Section in discussion draft was a placeholder and did not include any legislative language) |
Akros Pharma wants incentives for companies to develop companion diagnostic for targeted medicines. Suggests MODDERN Cures Act (H.R. 3116 from last Congress) approach. Would provide 12 months of additional exclusivity to companies that develop a companion diagnostic contemporaneously with a new drug and a six-month exclusivity extension on exclusivities for a diagnostic developed separately from the original product. Alzheimer’s Association feels new regulations on diagnostics must include protections for patients and consumers. Must be strong scientific consensus behind new Dx modalities and the results must be reported to patients in the proper context (e.g., differences between increased risk factors and diagnosis) |
|
NIH Federal Data Sharing (Title II, Subtitle L) |
|
|
Changes Requested |
GSK wants more clarity on what data must be shared and how confidential commercial information will be kept private. Also said anonymized individual patient level data would need to be shared through a controlled access system to ensure patient privacy and use of data for valid scientific research. |
|
Accessing, Sharing and Using Health Data for Research Purposes (Title II, Subtitle M) |
|
|
Compliments |
GSK says Section 13444 in particular is “key enabler of industry’s ability to collect a deeper understanding of disease states, treatment pathways and improved patient outcomes.” |
|
Longitudinal Study On Outcomes Of Patients With A Chronic Disease (Title II, Subtitle N) |
|
|
Compliments |
BIO: this is one of BIO’s two major priorities for 21st Century Cures. |
|
Criticism |
Alzheimer’s Foundation of America concerned that proposed study could shift resources away from promising research and divert focus from National Plan to Address Alzheimer’s disease; says existing longitudinal studies can provide similar data. |
|
Clinical Research Modernization (Title III, Subtitle A) |
|
|
Changes Requested |
Novartis said large multisite studies should be able to to use a small number of “lead” IRBs instead of just a single one if the circumstances necessitate. |
|
Bayesian Statistics And Adaptive Trial Design (Title III, Subtitle B) |
|
|
Criticism |
Lilly worries that “while discussion draft asks FDA to issue further guidance … it does not address other observed issues. Industry experience suggests that existing guidance is not being implemented consistently across the FDA, potentially due to lack of experience with these innovative approaches.” Industry and FDA lack clear process for discussing these design proposals and reaching consensus in a timely manner, Lilly said, sometimes leading to the decision to use traditional approaches instead. “Strengthening consistency of implementation either, by engagement of outside experts with relevant experience or through use of management systems that drive toward this consistent implementation, must be a high priority.” |
|
Changes Requested |
ACRO wants FDA directed to implement and administer enforceable development plans to prevent regulatory confusion during the course of a trial.
Genentech urges more resources and funds are needed to investigate new methods in order to gain a better understanding of benefits/challenges associated with their use.
AstraZeneca says FDA should include key external experts and stakeholder’s in the review and revision of the guidance documents on adaptive trial design and Bayesian methods. Options include a public meeting with stakeholders or another consultation process. |
|
Clinical Trial Modernization: Post-approval Studies And Trials (Title III, Subtitle C) |
|
|
Reaction |
Pro — AstraZeneca Con—Lilly |
|
Compliments |
AstraZeneca supports goals of section |
|
Criticism |
Lilly says focus is wrong. While periodically revaluating post-marketing requirements and commitments is useful, there are ways to accomplish this today. Instead, effort should be on avoiding rushed manner in which these studies are discussed and agreed to at end of application review period. “Opportunities to engage in a more thoughtful discussion of the scientific questions at hand and the most appropriate types of studies to answer each question before solidifying the requirements would be more valuable … and could potentially utilize mid and/or late cycle meetings that are part of PDUFA V NME review model.” |
|
Changes Requested |
AstraZeneca wants clarification that only sponsor may request evaluation of post-approval study or clinical trial under this section. Also wants to expand reasons for which a renegotiation of the design or timelines of a post-approval trial may occur. |
|
Pediatric Research Network (Title III, Subtitles D & E) |
|
|
Criticism |
ACRO says members have already created global pediatric trial networks. NIH should try and engage these private networks before building their own network from scratch. |
|
Vaccine Access, Certainty And Innovation (Title IV, Subtitle C) |
|
|
Reaction |
Pro — ACRO, GSK So-So — IDSA |
|
Compliments |
ACRO strongly supports efforts to accelerate innovative vaccine development.
IDSA likes guidance on vaccine development section, as well as modifications to the priority review voucher program for tropical disease, supports expanding vaccine research at NIH. |
|
Criticism |
IDSA is concerned that requiring faster review of vaccines by the Advisory Committee on Immunization Practices without any additional support to meet the requirements could jeopardize the integrity of ACIP’s recommendations by failing to provide sufficient time for a thorough review of relevant data. Review of transparency and consistency of ACIP process is unnecessary, IDSA says, because of extensive literature on issue. Recommendation could divert strained CDC resources from other activities that would have more significant impact on public health. |
|
Changes Requested |
GSK wants GAO study on impact of Medicare and Medicaid reimbursement levels on access to vaccines. |
|
Medicare Local And National Coverage Determinations (Title IV, Subtitle H) |
|
|
Changes Requested |
Novartis recommends deleting “national” from title of section to make clear it only applies to the Local Coverage Determination process. Lilly wants language directing CMS to ensure that existing National Coverage Determinations are not applied to new products to limit their coverage. |
|
Telemedicine (Title IV, Subtitle I) |
|
|
Changes Requested |
Alzheimer’s Foundation of America wants CMS to have authority to expand or constrict telehealth services according to their budget impact. Wants telehealth advisory committee to advise CMS on what services should be covered based on evidence of cost savings or cost neutrality. Genentech believes expanding Medicare coverage of telestroke services to geographical regions outside of rural areas would have significant benefit on outcomes of Medicare beneficiaries. |
|
Revise Medicare Inpatient Prospective Payment's New Technology Add-On Payment Reimbursement (Title IV, Subtitle J) |
|
|
Reaction |
So-So—Genentech and Amgen support NTAP provision, oppose NDC code provisions |
|
Compliments |
Amgen supports the creation of a process to appeal NTAP denials
Genentech supports need to increase transparency in NTAP decision-making process |
|
Criticism |
Genentech opposes replacing Healthcare Common Procedure Coding System (HCPCS) codes with National Drug Codes (NDC) under Medicare Part B due to directional rebate exposure, operational concerns and technical limitations of NDC. NDC does not make it easier to trace drug to manufacturers, Genentech says, and makes it operationally more difficult to apply rebates to physician-administered drugs. It also imposes operational burdens on customers, and hospitals have sued over requirement to report NDCs because of this burden. Amgen notes NDCs don’t have a mechanism for billing for doses that are less than the amount represented by the NDC, for products supplied in multi-packs providers may not have systems in place that track the NDC once the packs are opened and separated, creating a risk that an incorrect NDC would be reported. Amgen also worries the proposal opens up the part of the Medicare statue addressing Part B drug and biological payment, which is not necessary to address timeliness of new codes. |
|
Changes Requested |
Amgen suggests revisions to the NTAP appeal process such as NTAP applicants having the right to seek judicial review if dissatisfied with administrative review. Clarify that the adjudicator of NTAP appeals will be the HHS Department of Appeals Board.
Genentech wants newness criteria for NTAP potentially redefined to refer to the date of approval for each indication rather than the date of the first approved indication. Says this is especially important for rare disease treatments. |
|
Lowering Medicare Patients’ Out-Of-Pocket Costs (Title IV, Subtitle K) |
|
|
Compliments |
Genentech supports need to improve transparency in out-of-pocket costs but said more information is needed as proposal may have significant logistical and operational challenges given diversity of geographic locations and entity types in which services are provided and the reimbursement structure of different parts of Medicare. Also more, information is needed to ensure protection of proprietary information. |
|
Evaluation Of Provider Consolidation and Medicare Payments (Title IV, Subtitle M) |
|
|
Compliments |
Genentech supports with suggestions to strengthen. Supports need to understand existing and identify trends in provider consolidation, especially acquisition of independent practices by larger hospitals/systems. In addition to reviewing Medicare payment proposals to assess impact on provider consolidation, HHS should also assess whether the proposal would lead to greater out-of-pocket spending for Medicare beneficiaries. |
|
Medicare Pharmaceutical and Technology Ombudsman (Title IV, Subtitle P) |
|
|
Compliments |
Genentech supports and provides suggestions to strengthen so proprietary data is excluded from any information made public. |
|
Ensuring Local Medicare Administrative Contractors Evaluate Data Related To Category III Codes (Title IV, Subtitle Q) |
|
|
Changes Requested |
Novartis recommends adding language requiring contractors to cover these codes unless a formal explanation and rationale for non-coverage is provided. “Medicare’s contractors frequently deny Category III codes without any review of the merit for coverage and payment.” |
|
Continuing Medical Education Sunshine Exemption (Title IV, Subtitle S) |
|
|
Changes Requested |
Amgen says that while legislative text seems to support excluding anything that could be used for physician education from the Sunshine Act’s reporting requirements (including peer-reviewed journals, journal reprints, journal supplements and medical textbooks) the title of “continuing medical education” has a narrower meaning. Suggests revising the terminology to mitigate the risk of confusion. |
|
Exclusivity Extension For American-Manufactured Generics and Biosimilars (Title V, Subtitle A) |
|
|
Changes Requested |
GPhA says several questions need to be addressed before it takes a position on issue, including potential implications on free trade agreements and the efficacy of the provision in encouraging domestic manufacturing for manufacturers who do not file Paragraph IV challenges. |
|
21st Century Manufacturing (Title V, Subtitle B) |
|
|
Changes Requested |
Amgen submitted draft language that details the types of concepts which may help speed up the development of new medical products. Also said one year requirement for final guidance in this area is likely not feasible. Said it would be more beneficial to set up a process for a public meeting and proposed rule. GSK wants FDA to update its guidance regarding novel manufacturing techniques, to assure establishing an enabling regulatory environment to facilitate development and implementation of innovative pharmaceutical manufacturing approaches. |
