2020 Antibiotic Goal in Jeopardy? HHS Analysis Shows Need For Earlier, Larger Incentives
Executive Summary
Goal of 10 new antibiotics by 2020 may be unlikely given current marketplace for antibiotics, some experts predict as a new study shows recent proposals to revive development may be focused at wrong point in development timeline. Study suggests need for large increases in government funding.
The steps taken in the GAIN Act and even the proposed limited-population pathway under consideration won’t be enough to reinvigorate the antibiotic therapeutic area, a new government-commissioned study concludes, suggesting that a combination of new incentives and reforms are needed – ones aimed at the very beginning of the drug development program and likely to carry a hefty price tag.
The mechanisms deployed by GAIN and a limited-population pathway – increased exclusivity and reductions in trial size and time to market – may be able to achieve only minor to limited gains in encouraging the field compared to other approaches that could be deployed, explained Kevin Outterson, a health and corporate law professor at Boston University who specializes in antibiotic resistance. Outterson was an independent consultant on the HHS-commissioned study.
The call for a multitude of approaches to assist industry in developing and bringing new antibiotics to market was echoed on Capitol Hill by a similarly wide array of stakeholders from lawmakers to FDA, academics, small companies and big pharma at a June 19 conference on the antimicrobial marketplace sponsored by the industry-led Antimicrobial Innovation Alliance and the Infectious Diseases Society of America, which represents scientists and health care professionals.
The stakes are high, as stakeholders pointed out there are already bacterial infections for which there are no treatments. In 2010 IDSA spurred a global commitment to develop 10 new systemic antibacterial drugs by 2020, but Duke pediatrician and IDSA member Danny Benjamin said at the conference that “as it is looking right now, it is just not going to get there.”
He predicted five new antibiotics by 2020. “If we keep doing what we’ve been doing we are going to keep getting what we’ve been getting.”
GlaxoSmithKline PLC’s David Payne, head of the Antibacterial Discovery Performance Unit, agreed. “I don’t think it’s likely it would happen with where things are currently and the incentives as they are,” he told “The Pink Sheet.”
Payne added that “it’s less of a numbers game,” noting that what is critically important is the makeup of those molecules. If 10 drugs are developed but none address multi-resistant gram-negative infections, “that’s still very concerning,” he said.
Cubist Pharmaceuticals Inc., perhaps the most optimistic player in the field, said its goal is to contribute as many as four or five of the drugs to the 2020 goal but still acknowledged the challenges. “This year we are more likely to see a bump up [in approvals] given the nature of what’s in Phase II and Phase III; we think that will thin out in the years ahead unless something changes,” Tim Hunt, senior VP of public affairs, said in an interview.
Cubist, which already markets Cubicin (daptomycin) and Dificid (fidaxomycin), recently saw another new antibiotic approval with Sivextro (tedezolid) for acute bacterial skin and skin structure infections, joining Durata Therapeutics Inc.’s Dalvance (dalbavancin), which was cleared for the same indication in May (Also see "Cubist’s Sivextro To Beat Durata’s Dalvance To Antibiotic Market" - Pink Sheet, 23 Jun, 2014.). But the space remains challenging, as Basilea Pharmaceutica Ltd.’s recent halt to ceftobiprole development underscores (Also see "Basilea Ceftobiprole Saga Wages On; U.S. Development Ditched" - Pink Sheet, 25 Jun, 2014.).
One area that might hold more promise is development of vaccines and diagnostics. The study found incentives are not needed to stimulate the development of vaccines for acute bacteria otitis media (ABOM) or rapid point of care diagnostics for MRSA.
Numbers Dictate Re-Thinking Timing, Type of Incentives
“You may not be surprised to find that we are dramatically undervaluing these drugs. They are worth a lot more to society than we are currently allowing companies to capture. Which is why business models are broken at the moment,” Outterson told the conference.
But when and how society decides to reward antibiotic developers will be critical, according to the HHS study conducted by Eastern Research Group and made public June 17. Which incentives are offered to companies and when the incentives are offered is key to getting research off the ground, the researchers found.
Eastern Research Group developed an analytical decision-tree model framework to assess the impacts of different market incentives on the private and social returns to developing new antibacterial products. It found that currently, private returns associated with six types of antibacterial products at the beginning of preclinical research fell far short of a $100 million threshold.
The $100 million threshold was used because it is comparable to figures used in similar analyses and has been indicated as the tipping point for smaller companies to work in the field, according to experts interviewed for the study.
The six indications were:
- Acute bacterial otitis media (ABOM)
- Acute bacterial skin and skin structure infections (ABSSSI)
- Community-acquired bacterial pneumonia (CABP)
- Complicated intra-abdominal infections (CIAI)
- Complicated urinary tract infections (CUTI)
- Hospital-acquired/ventilator-associated bacterial pneumonia (HABP/VABP)
It found that the average net present value to the developer considering whether to start preclinical research ranges from a low of -$.4.5 million for HABP/VABP to a high of $37.4 million for CABP (see graph).
The 90% confidence intervals put all of the indications in the negative with HABP/VABP coming in at -$23.5 million. The upper bounds all did surpass the $100 million threshold, including a $126.7 million upper bound for HABP/VABP and $330 million for CABP.
Estimated Net Present Value To Sponsor Considering Starting Preclinical Research
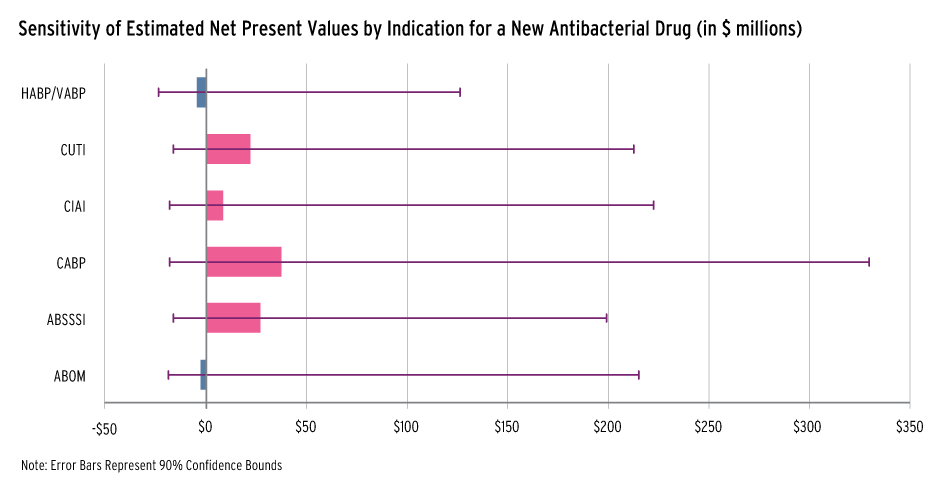
Source: Analytical Framework for Examining the Value of Antibacterial Products, HHS-commissioned study conducted by Eastern Research Group
In comparison, estimates on the average net present social value of developing drugs for these indications ranged from $486.6 million for ABOM to $12.2 billion for HABP/VABP. While the 90% confidence intervals did place two indications below the $100 million threshold (ABOM and ABSSSI), they are all much greater than the private net present value estimates.
GAINing A Little: IP, Exclusivity Extensions Insufficient
The study then looked at four incentives in the context of their model to calculate the level of each incentive that would be needed to achieve the $100 million estimated net present value for a sponsor at the start of preclinical phase.
Overall, it found that while it is difficult to ascertain the necessary levels of incentives needed, there were certain patterns that can assist policy makers (see graph).
How Much Incentive Needed To Reach $100 Million Threshold
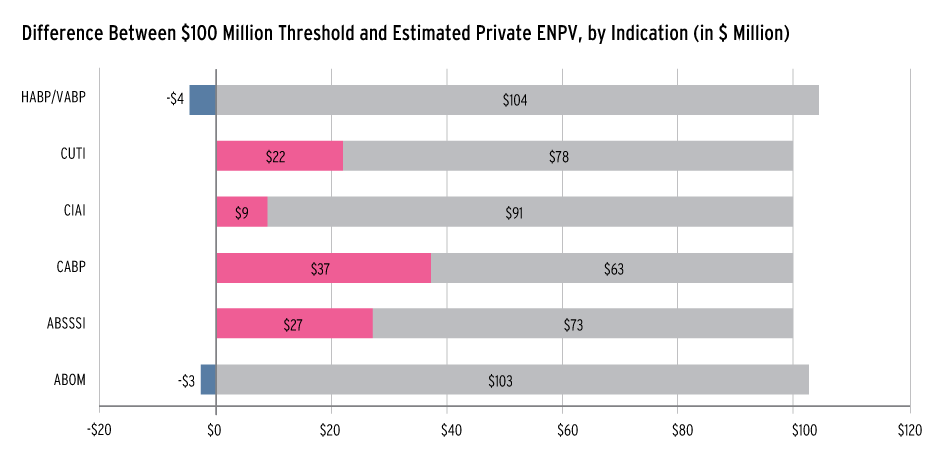
Source: Analytical Framework for Examining the Value of Antibacterial Products, HHS-commissioned study conducted by Eastern Research Group
For example, intellectual property extensions alone are not sufficient to incentivize a sponsor at the preclinical phase primarily due to the effect of discounting where the product revenues in future years contribute increasingly less to the net present value, the study said.
“IP extensions, exclusivity, marketing exclusivity have almost no positive effect on the model … When the company is making a decision whether to green-light a Phase I or Phase II project the IP landscape in year 21 through 25 is just so far down the road that the discounting severely impacts it. So it’s not going to be sufficient at all,” Outterson said.”
This model indicates the five years of additional exclusivity granted to certain antibiotics targeted under the Generating Antibiotic Incentive Now (GAIN) Act, which became law as part of the FDA Safety and Innovation Act in 2012, may not have an overwhelming impact on the therapeutic area (Also see "Antibiotic Development Incentives Face Critical Test As NDA Reviews Begin" - Pink Sheet, 20 Jan, 2014.).
It also signals that the DISARM (Developing an Innovative Strategy for Antimicrobial Resistant Microorganisms) Act’s more generous reimbursement formulas under Medicare, while helpful, might not be enough to spur initial development either (Also see "Antibiotic Incentive Legislation: Can Reimbursement Horn In?" - Pink Sheet, 25 Apr, 2014.). The DISARM Act has taken a back seat to the limited population-drug approval pathway (LPAD) approach proposed under the Antibiotic Development to Advance Patient Treatment (ADAPT) Act.
LPAD Also Not a Cure-All
Decreasing the overall time to market through modifications to the clinical trial process and approval standards is also insufficient for some indications such as ABOM, CIAI, and HABP/VABP, the analysis determined. For the other indications, the time to market would need to be reduced “significantly” to two to four years from current levels of nine to 10 years for ABSSI, CABP and CUTI.
This finding could dampen some of the enthusiasm surrounding the proposed ADAPT Act, which would allow for an antibiotic to be approved for a small population of patients with an unmet need based on smaller clinical trials. The LPAD approach, even when paired with GAIN, may still leave a lot to be desired.
“Reducing the time [to market] is valuable, but what was needed in order to bring things into the threshold was something in order of two to four years cut off in development time, so my question is whether that’s practical?” Outterson said. “So LPAD it will be great, especially for patients that will have access to the drugs more quickly but unless we are cutting two to four more years off, we have to see that as a small part of the bigger picture.”
“I don’t think ADAPT is enough but it’s clearly a move in the right direction,” Merck & Co. Inc.’s Brian Woolhouse, VP and general manager of acute care hospital, said in an interview.
The bigger picture, Outterson said, is reducing the cost of capital and then doing something about reimbursement once a drug comes to market with a large price tag.
Push Incentives More Cost Effective For Industry, Costly For Gov’t
Incentives from government sources, in terms of tax breaks or grants and the like, are an important piece, but should be increased and better timed, the researchers concluded.
A significant factor to consider regarding grants, awards and prizes is that the amount needed to incentivize companies to enter preclinical research increases if it is paid out at later stages of clinical development due to time discounting and the greater costs and risks of later-stage clinical research.
Providing financial support earlier on is usually more cost effective, said Michael Eichberg, senior director at Achaogen Inc., a clinical-stage biopharmaceutical company working on antibiotics for multi-drug resistant gram negative infections.
Early incentives, what Eichberg called “push incentives,” are preferable to “pull” incentives that are available post-market because every dollar from a push incentive goes to a company that is investing in R&D right now, immediately improving the net present value in a one to one ratio.
But the pleas for more and early government funding come during a period where budgets for key HHS agencies like NIH are remaining largely flat (Also see "NIH Director Ignores Proposed Funding Based On HHS Goals In Budget Request" - Pink Sheet, 26 Mar, 2014.).
More established companies like Merck may not be as reliant on the push incentives, Woolhouse noted.
“Our approach is that I don’t think it’s going to be a panacea of one type of incentive and I think that’s my key point,” he said. For a smaller company without a commercial presence, “there is a lot to risk [in] how they commercialize. They need everything they can around the push incentives because they don’t have anything in the other space. Our view is … it’s a much more coordinated approach between push, stewardship and pull incentives, [that]’s really where we want to focus our time and effort.”
The Eastern Research Group study found that reducing the cost of capital through various mechanisms such as tax incentives, grants or government contracts was helpful, but the level of incentives needed was quite high. “And really depressingly for four [indications], even a zero cost of capital didn’t do anything,” Outterson said, due to other costs associated with drug development such as building factories and promotion.
The impact of tax incentives to reduce the cost of capital did vary by particular indication, but the study found that for ABOM, CIAI and HABP/VABP even a zero cost of capital is insufficient to reach a $100 million expected net present value. In comparison, the percentage reduction in the cost of capital through tax incentives needed for ABSSSI was 33%, while CUTI would require an 81% reduction in cost of capital to reach the $100 million threshold.
Outterson advocated for fully refundable tax credits that put cash in the pockets of small companies. “It needs to be on the order of 50% of the cost of capital in order to make a big dent,” he said.
“The amount of grants over preclinical, Phase I, Phase II, Phase III and at registration that will be necessary to bring a product to market for that indication, with that $100 million threshold, is about $1 billion. It’s a big number,” Outterson added.
“So whatever we are spending on BARDA [Biomedical Advanced Research Development Authority] and the like today, the data here says we should be increasing that by a significant amount of money if we really want to have the sort of impact we are hoping to in this area.”
Outterson said we should be doubling the research budget of NIH and other basic science efforts over the next 10 years.
“Because you are spending today’s dollars in R&D and it takes between 10-15 years to get to market,” Merck’s Woolhouse explained, “in order to really transform and encourage the right business case to come through, the types of changes and impact that you need in the push piece are pretty significant because that’s where the big dollars are spent in discovery and then in Phase III and those are all in today’s dollars.”
No Private Value For Some Antibiotics
Some limitations to the Eastern Research Group study are that it does not reflect some recent regulatory flexibility. The model assumed two Phase III studies with 500 patients total and 6.9 years from Phase I to approval. More regulatory flexibility could cut the size and timeline of clinical programs, making for a better economic picture.
And it didn’t take into account antibiotics that might be sold for multiple indications, but as Outterson pointed out, there is a need for narrow spectrum treatment. It also didn’t deal with the multi-drug resistant organisms which would come with a smaller number of potential patients, though the impact on patients’ lives could give these drugs more value.
The model did not factor in some criteria drug developers use when making decisions about which compounds to pursue, such as peak-year sales value, return on R&D investment and expected return in comparison to other new product candidates as well as where the compound fits within their entire portfolio. These factors that could enhance net present value.
However, the model also didn’t take into account enhanced stewardship and conservation efforts that will slow sales and drive the private expected net present value down further.
It didn’t consider scenarios in which a drug is developed that may or may not be urgently needed in some future disaster.
“A drug that might save us against the apocalypse, might save us against the plague, the infection that kills 10 million people or something. Well it has no value until the infection epidemic hits,” Outterson said, meaning society has to decide if it is willing to pay for drugs that may not be used.
“Infectious diseases are renowned for their unpredictability; you never know what’s lurking around the corner with an infectious disease. But there is absolutely no incentive for any company to start working on a program for a future unmet medical need,” GSK’s Payne added.
Outterson suggested we think about an insurance type model for some of these drugs, an idea that is similar to models GSK has advocated where compensation for antibiotics would not be linked to number of prescriptions sold (Also see "Antibiotic Commercial Models Under Revision To Tackle Stewardship Tension" - Pink Sheet, 15 Jul, 2013.).
“It doesn’t bother you that you pay fire insurance and your house doesn’t burn down. We should be willing to pay for drugs that we are not going to use unless something horrible happens. We don’t have any system to do that currently,” Outterson said.
And these areas where private value may be the lowest may actually be some of the biggest public health threats.
“Let’s focus on the things that brought us to the party – it’s multi-drug resistant organisms that might kill us. It’s not really complicated urinary tract infections that bring us here today, although those are bad,” Outterson said. GAIN’s qualified infectious disease product standard actually sets a lower standard than priority review, he noted.
“I think we should focus on helping projects be green-lighted by companies in the early phase and we might want to reward things that are gram negative at a higher level or maybe not gram negative but things that the CDC identifies as the biggest problems.”
Engagement Is High – Will It Last For Multiple Solutions?
The saving grace for antibiotics may be that the momentum is in its favor, with governments in particular giving the topic attention.
On July 2, the British government and Wellcome Trust charity, which came out of GSK antecedent Burroughs Wellcome but is currently unaffiliated with GSK, announced an internationally-focused commission to study drug-resistant infections and assess how best to jump-start development of new antimicrobial medicines using innovative commercial incentives (Also see "Antibiotic “Market Failure” Getting Review By Wellcome Trust, British Gov’t" - Pink Sheet, 2 Jul, 2014.).
“I think global engagement in this problem is at an all-time high,” GSK’s Payne said. “It’s been sort of a concern for many decades but I think it’s hit a pinnacle now. I think the important part here is that if all of this engagement fails to result in some solid actions we will probably [have] some problems, but I remain optimistic that all of these discussions driven by such well-thought-of groups around the world will result in a better place in the future.”
Payne said he has never seen government interest at this level before and this has spurred his optimism “that the landscape is changed.”
One of the main tests may be how long-lasting the attention is as it becomes clear one or two fixes won’t solve the problem.
For example, Merck’s Woolhouse said of the DISARM Act, “It’s not a panacea … I think it’s a good step in the right way.” And he said that much of the AIA/IDSA conference discussions were around how many of the ideas to improve the antibiotic marketplace were not sufficient on their own.
Another issue will be gaining consensus and cooperation as different approaches are discussed.
So while GSK is focusing on a delinking payment model, Cubist is advocating for reimbursement reform that takes antibiotics out of the diagnosis-related group payment model where hospitals are reimbursed for antibiotic use as part of a fixed fee to manage a patient.
Getting reimbursed for the value of an antibiotic is a critical element, and “fixing this core problem caused by the DRG is the single best way to fuel global antibiotic innovation,” Cubist’s Hunt said.
