Drug Recalls Soared Again in 2013, Driven by Contamination
This article was originally published in The Gold Sheet
Executive Summary
FDA reported 1,276 drug recalls last year, the third-highest level ever. Inspections of pharmacies that compounded purportedly aseptic injectables drove the numbers, but pharmaceutical manufacturers also contributed as they struggled with particulates, precipitation and other issues.
U.S. drug recalls increased in 2013 to the third-highest level ever, driven by microbial and particulate contamination at pharmaceutical compounding and manufacturing facilities.
In all, there were 1,276 drug recalls last year, nearly twice the 655 recalls in 2012 and over half the 2,329 recalls FDA reported in the peak year, 2011.
Recall specifics
An Excel file readers can download here gives details about all of last year’s 1,276 drug recalls. The spreadsheet can be filtered by any of its 11 columns of data, for example to focus on individual companies, problem areas or dosage forms involved.
With two exceptions, problem areas hewed close to historical levels level last year. The exceptions:
- There were 873 contamination-related recalls, more than three times the prior 10-year average; and
- There were just 103 recalls resulting from GMP failures, half the average over the prior decade.
Similarly, most dosage forms were recalled at rates close to the 10-year historical average with a few minor exceptions – and one major exception:
- Injectables recalls were more than seven times the average;
- Ophthalmics recalls were twice the average;
- Nasal product recalls were three times the average; and
- Oral solids recalls were just two-thirds the average.
Problems with contamination increased last year
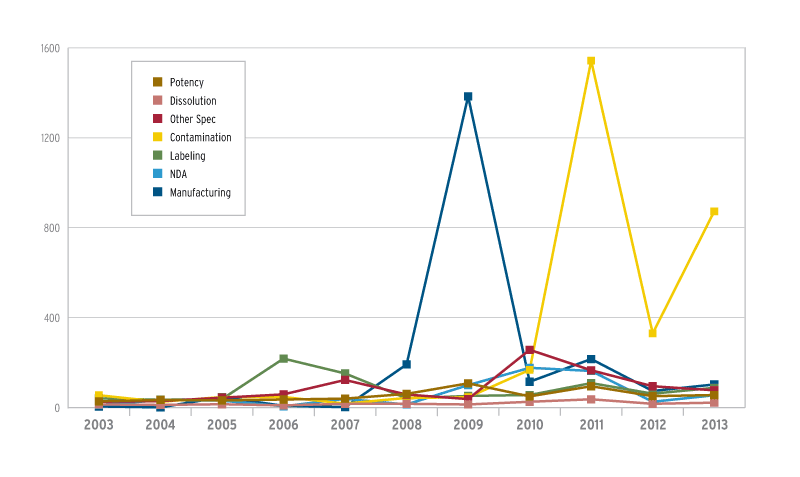
Drug recalls by problem area
Small percentage sourced abroad
Just 65 of last year’s recalls were identified in FDA’s enforcement reports with manufacturing facilities outside the U.S., or about 5.1% of the total. In some cases, a country of origin was given but without any information about the company or plant. There are probably many cases where foreign manufacturers or sites were simply not disclosed.
Of the 65 foreign-sourced recalls, 21 were for products from plants in India, 17 from Canada, six from Germany, three from Belgium, two each from Australia, Austria, Mexico, and one each from China, the Czech Republic, Israel, Italy, Jamaica, Jordan, Poland, South Korea, Spain, Switzerland, Turkey and the United Kingdom.
Types of contamination seen
Of last year’s contamination-related recalls, those related to microbial contamination were the most numerous, most likely to be serious, and most likely to proceed without any confirmation of actual contamination.
There were 776 recalls involving microbial contamination, compared to 89 involving particulates and just eight resulting from cross-contamination of drug products.
All but two of the 89 particulates recalls involved actual contamination, though just eight were rated Class I. Similarly, all but one of the eight cross-contamination recalls involved actual contamination, and none were rated Class I.
FDA reserves its Class I designation for recalls that likely pose a risk of serious adverse health consequences or death.
But it was different with microbial contamination, which can be hard to see, can spread after it’s missed, and can pose serious, immediate health risks. For these reasons, FDA focuses a lot on sterility assurance during inspections, and manufacturers that cannot provide that assurance to the agency often find themselves recalling product.
Only 328 of the 776 micro recalls involved actual contamination, but those cases include all 14 Class I micro recalls, which in turn comprise nearly two thirds of last year’s 22 Class I contamination recalls – and 20% of all 71 Class I recalls.
Pharmacies were at the heart of last year’s surge in contamination recalls. Compounding pharmacies accounted for 709, or 81% of the year’s 873 contamination-related recalls, including 313, or 95% of the 328 recalls that involved actual contamination, and seven (32%) of the 22 Class I recalls.
The other 15 Class I contamination recalls involved confirmed bacteria, mold, yeast, brass, glass particulates, fibrous material and other apparently un-indentified particulates at pharmaceutical manufacturers.
A focus on sterile compounding
Increases in contamination-related injectables and ophthalmics recalls resulted from the attention FDA investigators lavished upon compounding pharmacies early last year following a September 2012 outbreak of fungal meningitis traced to massive sterility failures at the New England Compounding Center in Framingham, Mass. (Also see "What FDA Saw at Compounder Behind Fungal Meningitis Outbreak" - Pink Sheet, 29 Nov, 2012.).
The Centers for Disease Control and Prevention has linked that outbreak to 751 medical cases in 20 states, including 64 deaths.
In early 2013, FDA inspected more than 50 other pharmacies that also compounded large quantities of sterile injectables in operations that were more like manufacturing than traditional compounding. FDA found a lack of sterility assurance at these pharmacies, and many conducted recalls as a result of the inspections.
Last year’s weekly FDA enforcement reports are peppered with these recalls, many of them involving dribs and drabs of vials and syringes filled with an unusual array of products manufacturers don’t make.
Enough of these reports appeared in 2012 for injectables recalls to eclipse oral solids recalls for the first time. There were 234 injectables recalls that year compared to 229 for oral solids.
Last year’s bolus of FDA pharmacy inspections boosted 2013 injectables recalls to 782, compared to just 306 for oral solids.
Injectables far exceeded oral solids in last year's recalls
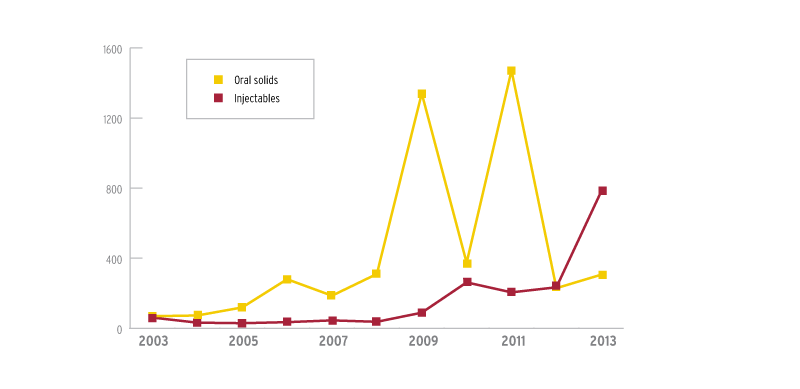
Drug recalls by top two dosage forms
Impact of inspections varied
The impact of those inspections on last year’s recall totals varied significantly. There were two reasons: The agency took different approaches to counting individual recalls in each compounding recall event and some compounders refused to recall products.
When Main Street Family Pharmacy in Newbern, Tenn., recalled all products six days into an inspection last May, FDA counted it as 298 recalls – more than one third of last year’s contamination-related drug recalls. But when Med Prep Consulting in Tinton Falls, N.J., recalled all products three days after an inspection began last March, FDA counted just two recalls – one for more than 400,000 IV bags of magnesium sulfate and the other for everything else (Also see "Clean Room Lessons from FDA’s Compounding Pharmacy Inspections" - Pink Sheet, 27 Jun, 2013.).
FDA lacks legal authority to make companies recall drug products, and there were cases last year where the agency was simply unable to persuade pharmacies to conduct recalls. Custom Compounding Centers in Los Alamitos, Calif., did not recall any product despite an inspection, a teleconference and a warning letter. Foundation Care of Earth City, Mo., and IV Solutions of Lubbock, Texas, rebutted their 483 reports point by point and recalled nothing.
FDA had to obtain warrants to inspect Lowlite Investments in Orlando, Fla., which does business as Olympia Pharmacy, and Wedgewood Village Pharmacy in Swedesboro, N.J., neither of which recalled any human drug products despite significant 483 findings.
Olympia has since registered as an FDA-regulated outsourcing facility and retained the services of Kymanox, a Highland Park, Ill., firm that provides quality, regulatory compliance and other services to pharmaceutical companies.
Wedgewood conducted limited recalls of veterinary drugs in March and June 2013, but still has not recalled any human drug products despite a Feb. 21, 2014, FDA warning letter that underscored the sterility assurance findings of the inspection, completed a year earlier.
Struggles with particulates
Particulates contamination bedeviled many pharmaceutical manufacturers last year.
Particulates had emerged as a major source of recalls in 2011 due to FDA concerns about problems with glass vials and syringes such as cracking and delamination (Also see "Contamination Issues Drove Drug Recalls to Record Levels in 2011" - Pink Sheet, 28 Mar, 2012.) (Also see "Glass Quality Crisis Prompts Multi-Faceted Array of Risk-Based Improvements" - Pink Sheet, 1 Aug, 2011.).
There were 33 such recalls that year, and some of them were rather large. For example, Centocor Ortho Biotech Inc., Horsham, Pa., on Sept. 22, 2010, recalled nearly 17 million vials of Amgen Inc.’s Procrit (epoetin alpha) due to the presence of glass lamellae, and that Oct. 13 expanded the recall to an additional 2.2 million vials. That Nov. 30, Amgen also recalled nearly 4.5 million vials of Epogen (epoetin alpha) for the same reason.
There were an additional 31 recalls involving other types of particulates in 2011, including polyvinyl chloride, stainless steel, rust, metal, wood, blue plastic, small rubber particles and food grade gasket materials.
In 2012, there were just 37 recalls involving particulate contamination, including nine with glass, two with silicone, one with stainless steel, one with metal shavings and 24 that didn’t confirm the type of particulate, although some mentioned possible fibers, crystallization, charcoal, water and dirt.
In 2013, particulate recalls increased to 89, including 40 due to contamination with oil or chemicals, 12 involving metals, nine involving glass and four due to fibers or fibrous materials.
Particulates proliferated at West-Ward
FDA reported 20 recalls last year from West-Ward Pharmaceuticals Corp.’s Eatontown, N.J., facility, including 12 throughout the year that resulted from various types of particulate contamination.
The plant’s problems with particulates came to the fore during an FDA inspection that ran from July 9 to Aug. 28, 2012.
The first two observations of the seven-point Form 483 report focused on particulates.
FDA said West-Ward had failed to thoroughly investigate the presence of black particulates in at least 12 batches from seven products despite multiple product holds and market complaints since August 2011.
Batches of prednisone tablets and Lisinopril and hydrochlorothiazide were still on the market, FDA said, despite complaints months earlier and analytical findings of trace metal particles or metallic characteristics.
Investigations were still ongoing even though the company had already passed the deadlines in its standard procedures for handling product complaints.
FDA also criticized West-Ward’s handling of several particulate-tainted batches of ethambutol tablets. Microscopic and chemical analysis indicated the particles consisted of charred material. Unable to ascertain the root cause of the problem, West-Ward coated the affected tablets to cover up the dark spots and released them to the market, FDA said.
In another case, the company put several lots of prednisone tablets and Lisinopril tablets on hold in early 2012 to investigate contamination. The company concluded the contaminant was food grade lubricant, but didn’t substantiate its determination with any analysis, FDA said. West-Ward then culled the contaminated tablets and released the rest of the lots.
Another lot of Lisinopril remained on the market despite a “foreign matter” complaint and an investigation FDA said was incomplete. It only focused on testing and concluded that clumps found with the tablets were a combination of Lisinopril blend and food-grade lubricant.
The company designated two other lots of products for destruction after finding black spots, but should have conducted a root cause investigation first, FDA said.
There was also a problem with blue plastic appearing in multiple lots of amoxicillin powder for oral suspension, FDA said. There were six complaints in February and April 2012, but the company didn’t file a field alert about it until the inspection.
Recalls resulting from these and other particulates issues began in December 2012, and continued into November 2013.
There were a number of recalls due to uncharacteristic black spots. Some were identified as food grade lubricant with trace amounts of foreign particulates and stainless steel inclusions, others as steel corrosion with degraded tablet material and hydrocarbon oil with trace amounts of iron. There were black specks comprised of degraded organic material. There were gray defects.
Even West-Ward’s contract manufacturers had similar problems. The company had to recall more than 7,000 bottles of ciprofloxacin tablets made by Hikma Pharmaceuticals PLC, Amman, Jordan, after receiving a complaint about rubber-like material in a tablet. Also recalled were more than 20,000 bottles of carisoprodol tablets made for West-Ward by Shasun Pharmaceuticals Ltd. in Pondicherry, India, after heavy metals were found during routine stability testing.
Although West-Ward did not find the root cause of its wide range of particulates recalls, it is possible that an FDA warning letter from the year before might provide a hint.
That Feb. 3, 2012, warning letter, based on FDA’s previous inspection of the Eatontown plant in June 2011, focused primarily on variability of in-process material and drug product. West-Ward was having difficulties at the time keeping tablet thickness and hardness within specifications.
FDA had some issues, for example, with the way West-Ward resolved a problem with overweight digoxin tablets by running them through a tablet sorter, particularly given that digoxin is a product with a narrow therapeutic range.
The company received a close-out letter from FDA on March 26, 2014, indicated it had resolved the concerns raised in the warning letter. Whether the company has fixed the root causes of its recent recalls remains to be seen, but so far this year there has not been another.
Physician samples recalled
Novartis Pharmaceuticals Corp.’s Suffern, N.Y., site took back 4.7 million physician sample bottles of tablets in 15 Class II recalls on Aug. 8 due to contamination with Darocur 1173, a photocuring agent used in inks on shrink-wrap sleeves.
Recalls of physician samples are rare, but last year there were 21, and they resulted from five different events involving five different companies. In addition to the 15 Novartis recalls, Stayma Consulting Service, Suwanee, Ga., recalled nearly 6,000 physician samples of three products due to subpotency; Ascend Therapeutics US LLC of Herndon, Va., recalled nearly 5,000 defective pump bottles of a topical hormone therapy; Warner Chilcott PLC recalled nearly 190,000 physician sample bottles due to the presence of degradation products in tablets; and Valeant Pharmaceuticals International Inc. recalled 45,000 incorrectly labeled physician sample tubes of a topical acne treatment.
Investigational recalls seen
Another rarity: Recalls of investigational materials. Last year FDA reported two. Genentech Inc. July 30, 2012, recalled 2,140 trastuzumab kits because of the potential for glass particulates in diluent vials packed with the kits. And Pacira Pharmaceuticals Inc. of San Diego, Calif., recalled 324 vials of its investigational drug Exparel (bupivacaine liposome injectable suspension) due to subpotency.
It will be important to watch whether recalls of investigational materials increase as manufacturers increasingly rely on pharmacies to compound clinical materials (Also see "Risk and Reward: Pharmacy Compounding of Clinical Materials" - Pink Sheet, 27 Feb, 2014.).
Ingredients for failure
There were a number of recalls last year due to issues with ingredients. Most of the problems were with active pharmaceutical ingredients, but there were some due to excipients as well.
There were two cases involving recall of ingredients rather than drug products.
Church & Dwight Co. Inc. of Princeton, N.J., in June recalled 749 tons of Arm & Hammer sodium bicarbonate powder due to stainless steel and other contamination.
Also that month, Bayer HealthCare Pharmaceuticals AG of Wayne, N.J., recalled nearly 200 kilograms of medroxyprogesterone acetate API in six-kilogram drums because it failed on particle size at the 60-month stability specification test point.
Additionally, Boehringer Ingelheim Roxane Inc., Columbus, Ohio, on Aug. 30 recalled more than 15 million SPIRIVA/HandiHaler (tiotropium bromide inhalation powder) capsules due to the potential for extrinsic foreign particles in the API.
Boehringer Ingelheim GMBH made the capsules at its plant in Ingelheim am Rhein, Germany, which had received an FDA warning letter that May 6 that focused on foreign particles in an unnamed API.
In another case, Mylan Pharmaceuticals Inc. of Morgantown, W.Va., on Sept. 13 launched 12 recalls of tablets and capsules of amlodipine besylate, benazepril, lamotrigine and ciprofloxacin because the active ingredients had not been manufactured in accordance with good manufacturing practices.
Precipitation events
But the most common issue behind API-related recalls was precipitation.
In a pair of Class I recalls, Apotex Inc. of Weston, Fla., recalled over 37,000 pharmacy bulk package vials of piperacillin and tazobactam for injection in March and another 52,000 additional in May because of the product’s potential to precipitate and crystallize in IV bags or lines.
The contract manufacturer involved was a Hospira Inc. facility in Irungattukottai, India. An October 2012 FDA inspection of that plant resulted in a May 2013 warning letter, but it focused on sterility assurance (Also see "Contamination and Raw Material Testing Issues Raised in FDA Warning Letters" - Pink Sheet, 27 Sep, 2013.).
[Aaron Industries Inc.], of Lynwood, Calif., recalled nearly 43,000 bottles of over-the-counter cough/cold products in January and another 27,000-plus in April because one of the active ingredients, guaifenesin, had precipitated in some of the bottles.
AstraZeneca Pharmaceuticals LP, Wilmington, Del., recalled more than 80,000 vials of Merrem (meropenem) intravenous antibiotic manufactured by ACS Dobfar SPA Viale Addetta, Tribiano, Milano, Italy, because of the presence of precipitate, which meant there was a potential for incomplete constitution upon addition of diluent.
In another case, Fresenius Kabi AG, Schaumburg, Ill., recalled nearly 24,000 vials of irinotecan HCl for injection because API was precipitating in the product solution.
Failed media fills
There were three recalls in two events last year due to failed media fills. In one event, Gilead Sciences Inc. identified bacterial contamination in some media fill units during a routine simulation of the manufacturing of the injectable antifungal AmBisome, at its San Dimas, Calif., facility, and recalled nearly 500,000 vials of the product for lack of sterility assurance. Gilead also alerted a customer, Astellas Pharma US Inc. of Northbrook, Ill., which recalled 133,550 additional vials.
In another case, Nephron Pharmaceuticals Corp. recalled more than 17 million vials of albuterol sulfate inhalation solution after discovering that bacteria had grown in a number of media fill vials.
Manufacturing problems
Manufacturing issues have waxed and waned over the years as a problem area for recalls, peaking in 2009 with 1,384, mainly due to GMP shortfalls at Advantage Dose, a Shreveport, La., repackager.
Last year’s 103 manufacturing recalls were somewhat low, compared to recent years. One reason could be FDA’s emphasis last year on inspecting compounding pharmacies. Although many pharmacies don’t comply with the GMP regulations, there’s some question about whether they have to.
Those inspections generated a lot of recalls of sterile injectables due to lack of sterility or lack of sterility assurance. As might be expected, contamination was by far the dominant problem area cited in recalls of injectables and ophthalmics, the two most common dosage forms recalled by pharmacies last year, while there were hardly any manufacturing cites for injectables and none for ophthalmics.
Nevertheless there were some noteworthy manufacturing-related drug recalls last year.
There was just one Class I recall due to manufacturing issues. After a pharmacist discovered that three warfarin tablets in a 1,000-count bottle were oversized, Zydus Pharmaceuticals (USA) Inc. of Pennington, N.J., on May 13 recalled 960 bottles containing 1,000 2-mg warfarin tablets each that Cadila Healthcare Ltd., of Ahmedabad, India, had manufactured.
There was also a large recall on April 1 by Lloyd Inc. of Iowa, which recalled 90 million levothyroxine tablets and an additional 430,000 bottles of levothyroxine tablets.
FDA counted 20 recalls in that recall event, which occurred during a March 18 through April 16 FDA inspection of the facility.
Lloyd decided to undertake the massive Class II recall after a quality review of stability failures in previous lots, when the Shenandoah, Iowa, company concluded that it lacked enough data to determine that other lots were not affected.
Two of the 88 labeling/packaging recalls were rated Class I. They were a pair of labeling mix-ups by Advance Pharmaceutical Inc., Holtsville, N.Y., that led Rugby Laboratories Inc. of Duluth, Ga., to recall more than 16,000 bottles of acetaminophen labeled as aspirin and nearly 20,000 bottles of meclizine HCl for motion sickness labeled as ferrous sulfate iron supplement.
Also, Novartis Consumer Health Inc., Lincoln, Neb., had several large recalls due to poorly sealed tablet pouches and illegible or missing lot or expiration numbers. That facility also had some major recalls the year before due to foreign tablets resulting from line clearance failures that persisted due to poor complaint and root cause investigations (Also see "Contamination, Mix-Ups Drive Up Drug Recall Totals for 2012" - Pink Sheet, 30 May, 2013.).
Other specifications
Four of the 77 recalls grouped under the catch-all “other specifications” category were rated Class I. Two resulted from the sight of visible carboplatin crystals during a retain sample inspection at Hospira, Lake Forest, Ill. The other two resulted from a finding that piperacillin and tazobactam manufactured by Hospira Healthcare India Lvt. Ltd., Irungattukottai, India, could crystallize in IV bags or lines during reconstitution.
Potent threats
Two of the 58 potency/content uniformity recalls were rated Class I. Both involved superpotent hydrocodone bitartrate and acetaminophen tablets made by Qualitest Pharmaceuticals, Huntsville, Ala. Qualitest attributed one recall to a complaint. A customer, Mylan Pharmaceuticals Inc., Morgantown, W.Va., attributed the other to superpotent assays of both components.
More drug-tainted supplements
Of 55 recalls in the NDA category, 40 were rated Class I. These recalls were conducted by distributors of supplements that tested positive for prescription drug products. They resulted from FDA’s ongoing laboratory testing program, which continues to find sexual dysfunction drugs and their analogs, as well as weight-loss drugs and other active pharmaceutical ingredients in colorfully named products sold over the counter or the Internet. One product that FDA targeted last year is Reumofan Plus, an arthritis supplement that contains a non-steroidal anti-inflammatory drug, a corticosteroid and a muscle relaxant.
Pfizer’s site-change recall
A Class II recall event in the NDA category last September resulted from an unusual problem.
The dissolution profile of brand and generic spironolactone manufactured by Pfizer Inc. and its generics unit, Greenstone Healthcare Solutions, had changed after they started making it at a different manufacturing site. The problem was that there was no approved application supporting the alternative manufacturing site.
Summary tables and full details
The summary table below provides an analysis of last year’s drug recalls by dosage form for each market type, problem area and recall class.
Additional tables below put last year’s drug recalls into the context of the prior decade, both in terms of problem areas and dosage forms.
A separate story in this issue provides key facts at a glance for every one of last year’s 1,276 drug recalls
(Also see "Drug Product Recalls in 2013 Categorized by Problem Area" - Pink Sheet, 30 May, 2014.).
Find answers in recalls spreadsheet
An Excel file readers can download here gives even more information and can be filtered by any of its 11 columns of data, for example to focus on individual companies, recall reason codes or dosage forms involved.
Click here to open the Excel spreadsheet of 2013 drug recalls.
For each recall, the table says:
- What product was recalled and, if available, how much of it was recalled;
- What company manufactured it and recalled it;
- How seriously FDA rated the health threat the recalled product posed; and
- What reason the company gave for recalling the product.
The recalls are grouped into seven categories based on the reasons for them, starting with the most common reason and ending with the least common: Contamination; manufacturing/testing methods; labeling/packaging mix-ups; other product specifications; potency/content uniformity; compliance with NDA/monograph requirements; and dissolution.
In each category, recalls are listed by class, and in each class, by the date the recall began, which can precede FDA’s recall report by weeks or months.
|
2013 Drug Recall Analysis by Dosage Form |
|||||||||||||
|
2013 Drug Recalls |
Market Type
|
Problem Area
|
Recall Class
|
||||||||||
|
Dosage Form |
Rx |
OTC |
Con |
Man |
Lab |
Oth |
Pot |
NDA |
Dis |
I |
II |
III |
|
|
Inj |
782 |
782 |
|
756 |
7 |
4 |
10 |
5 |
|
|
23 |
749 |
10 |
|
OS |
306 |
216 |
90 |
62 |
57 |
35 |
43 |
35 |
52 |
22 |
44 |
185 |
77 |
|
Top |
64 |
23 |
41 |
5 |
4 |
36 |
5 |
12 |
2 |
|
|
21 |
43 |
|
OL |
57 |
17 |
40 |
4 |
26 |
9 |
13 |
4 |
1 |
|
1 |
42 |
14 |
|
Op |
33 |
29 |
4 |
32 |
|
1 |
|
|
|
|
2 |
31 |
|
|
Nas |
13 |
|
13 |
2 |
8 |
3 |
|
|
|
|
1 |
9 |
3 |
|
Inh |
8 |
7 |
1 |
5 |
|
|
2 |
1 |
|
|
|
4 |
4 |
|
Exc |
5 |
|
5 |
5 |
|
|
|
|
|
|
|
5 |
|
|
MW |
3 |
|
3 |
2 |
|
|
1 |
|
|
|
|
|
3 |
|
Tran |
3 |
3 |
|
|
1 |
|
2 |
|
|
|
|
1 |
2 |
|
API |
1 |
1 |
|
|
|
|
1 |
|
|
|
|
|
1 |
|
Ot |
1 |
1 |
|
|
|
|
|
1 |
|
|
|
|
1 |
|
Total |
1276 |
1079 |
197 |
873 |
103 |
88 |
77 |
58 |
55 |
22 |
71 |
1047 |
158 |
|
* The dosage form categories (and, in parentheses, their abbreviations) are: injectable (inj), oral solid (os), topical (top), oral liquid (ol), ophthalmic (opth), nasal (nas), inhalable (inh), excipient (exc), mouthwash (mw), transdermal (tran), active pharmaceutical ingredient (API), and otic. |
|||||||||||||
|
** The problem areas (and in parentheses, their abbreviations) are: contamination (con), manufacturing/test methods (mfg), labeling/packaging (lab), other product specifications (oth), potency/content uniformity (pot), compliance with NDA/monograph requirements (nda), and dissolution (dis). |
|||||||||||||
2003-2013 Recalls by Problem Area and Dosage Form
The following tables list the number of drug recalls for last year, the previous decade and the average over the previous decade by problem area and dosage form.
|
PROBLEM AREA * |
||||||||
|
Year |
Total |
Potency/ content uniformity |
Dissolution |
Other Product Specification |
Contamination |
Labeling/ packaging |
NDA/ mono-graph com-pliance |
Manu-facturing/ testing methods |
|
2013 |
1276 |
56 |
22 |
77 |
873 |
88 |
55 |
103 |
|
2012 |
655 |
51 |
17 |
95 |
330 |
62 |
26 |
74 |
|
2011 |
2329 |
95 |
37 |
166 |
1543 |
109 |
163 |
216 |
|
2010 |
849 |
50 |
26 |
257 |
168 |
56 |
177 |
115 |
|
2009 |
1742 |
108 |
14 |
38 |
46 |
52 |
100 |
1384 |
|
2008 |
426 |
61 |
17 |
58 |
43 |
41 |
14 |
192 |
|
2007 |
391 |
40 |
17 |
123 |
18 |
152 |
39 |
2 |
|
2006 |
384 |
36 |
10 |
59 |
47 |
218 |
6 |
8 |
|
2005 |
254 |
32 |
15 |
44 |
47 |
38 |
31 |
47 |
|
2004 |
170 |
34 |
12 |
29 |
26 |
35 |
33 |
1 |
|
2003 |
181 |
28 |
14 |
26 |
55 |
41 |
12 |
5 |
|
2003-2012 ave. |
738 |
54 |
18 |
90 |
232 |
80 |
60 |
204 |
|
* The problem areas (and in parentheses, their abbreviations) are: potency/content uniformity (pot); dissolution (dis); other product specifications (oth); contamination/sterility assurance problems (con); labeling/packaging mix-ups (lab); compliance with NDA/monograph requirements (nda); and manufacturing/testing methods (man). |
||||||||
|
DOSAGE FORM* |
|||||||||||||
|
Year |
Total |
OS |
Inj |
OL |
Top |
Op |
API |
Inh |
Nas |
Sup |
Ot |
Tran |
OHP |
|
2013 |
1276 |
306 |
782 |
57 |
64 |
33 |
6 |
8 |
13 |
0 |
1 |
3 |
3 |
|
2012 |
655 |
229 |
234 |
49 |
59 |
45 |
3 |
8 |
4 |
0 |
1 |
13 |
10 |
|
2011 |
2329 |
1472 |
205 |
172 |
423 |
5 |
2 |
6 |
7 |
12 |
7 |
8 |
10 |
|
2010 |
849 |
374 |
262 |
99 |
48 |
23 |
8 |
6 |
10 |
1 |
5 |
11 |
2 |
|
2009 |
1742 |
1341 |
89 |
169 |
53 |
23 |
0 |
7 |
3 |
30 |
5 |
14 |
8 |
|
2008 |
426 |
310 |
37 |
35 |
13 |
2 |
0 |
4 |
1 |
1 |
4 |
14 |
5 |
|
2007 |
391 |
186 |
45 |
54 |
30 |
37 |
0 |
4 |
0 |
2 |
7 |
6 |
20 |
|
2006 |
384 |
280 |
35 |
28 |
21 |
8 |
3 |
4 |
1 |
0 |
0 |
3 |
0 |
|
2005 |
254 |
119 |
29 |
37 |
27 |
13 |
2 |
6 |
4 |
11 |
1 |
4 |
1 |
|
2004 |
170 |
73 |
32 |
18 |
15 |
9 |
2 |
4 |
5 |
4 |
1 |
3 |
2 |
|
2003 |
181 |
69 |
61 |
16 |
14 |
8 |
6 |
4 |
1 |
1 |
0 |
1 |
0 |
|
2002-2011 ave. |
738 |
445 |
103 |
68 |
70 |
17 |
3 |
5 |
4 |
6 |
3 |
8 |
6 |
|
* The dosage form categories (and in parentheses, their abbreviations) are: oral solid (os), injectable (inj), oral liquid (ol), topical (top), ophthalmic (op), active pharmaceutical ingredients and excipients (api), inhalable (inh), nasal (nas), suppository (sup), otic (ot), transdermal (tran), and oral health products (ohp), which include toothpaste, mouth spray, and mouthwash. |
|||||||||||||
