Trade Groups Tutor Firms On Protein-Content Calculations
This article was originally published in The Tan Sheet
Executive Summary
AHPA and CRN worked together on guidance for doing the nutrient math correctly for protein-content labeling on supplement products. The groups recommend that calculations of protein content in nutrient labeling include only proteins that consist of a chain of amino acids connected by peptide bonds.
Determining protein content for dietary supplement labeling is rife for miscalculation, and the Council for Responsible Nutrition and American Herbal Products Association offer guidance on doing the nutrient math correctly.
The two trade groups worked together on guidance for calculating protein content stated on supplement product labels. CRN’s guidance, published April 30, also targets functional food labeling, while APHA’s version, published April 1, identifies the broader category of food products as an additional target.
The guidances recommend that calculations of protein content in nutrient labeling include only proteins that consist of a chain of amino acids connected by peptide bonds. Nitrogen-based nutrients that are frequently stated as protein content on supplement product labels are excluded in these calculations.
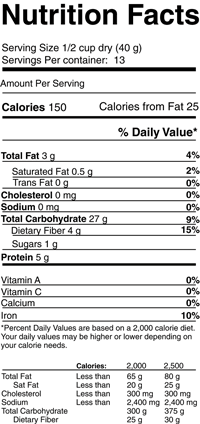
Formatting common for stating information on dietary supplement labels.
AHPA noted in its guidance announcement that many supplement firms already use the recommended quantification method and the Silver Spring, Md.-based organization encourages others to adopt the standard (Also see "In Brief: DXM, NPLEx, AHPA, Merck, Novel Ingredients" - Pink Sheet, 7 Apr, 2014.).
"This guidance highlights the industry's ability to identify an issue and collaborate to develop an effective solution. Members of AHPA's Sports Nutrition Committee have committed to adopting this guidance to help ensure food and supplement labels provide consumers with comparable information needed to make informed purchasing decisions," said President Michael McGuffin in AHPA’s April 1 announcement.
While FDA labeling regulations do not prohibit including non-protein nitrogen-containing substances in the calculations, the trade groups’ guidelines advise not to include these substances within total protein content on product labels. FDA regulations allow the amount of protein in supplements and foods to be calculated as a factor of nitrogen content, but do not define the sources of nitrogen that can be included in those calculations.
CRN President Steve Mister said FDA regulations leave room for a firm to state on a label more protein content than a product actually contains.
“We’re trying to get ahead of this issue,” Mister said.
The supplement industry should want to at least stay abreast of the issue. Products with protein content overstated on labels could “become the target of lots of product liability lawsuits,” Mister said.
Nutrient content claims are among the top targets in the consumer health care product space for class action claims (Also see "Top 10 Trends In Food, Supplement Labeling Litigation" - Pink Sheet, 18 Feb, 2013.).
Sports nutrition products are most likely to overstate protein content. And with sports nutrition the fastest growing sector of the overall supplement market, the number of products available in the U.S. with protein-content labeling also likely is growing.
Mister said CRN members and other firms that adhered to the CRN and APHA guidances for protein labeling could set a standard that those firms expect the rest of the industry to follow. While inaccurate protein-content labeling in supplement product might not attract regulatory attention, the practice could be a target of “self-policing in the industry,” he said.
“Companies that are doing this right down the road may end up calling out companies that are not doing it right,” he said.
