First-In-Class Drugs Are True Barometer Of Industry Innovation, FDA Says
Executive Summary
The agency suggests that categorizing approved drugs by level of novelty and tracking approvals by category is a better metric than NME count and should be the basis for assessment of the programs created by FDASIA to speed development of innovative products.
Not all new molecular entities are created equal, a team of FDA researchers is arguing, and so a more nuanced metric should be used to measure the outflow of innovative products from industry and the agency.
The agency has released a new analysis that categorizes drugs approved between 1987 and 2011 by their level of innovation – which shows that the level of truly novel medicines has remained stable, in contrast to the peaks and valleys suggested by annual tallies of all novel therapies.
The analysis, published in the August issue of Health Affairs, was authored by researchers at the Office of Planning, including Michael Lanthier, an operations research analyst; Kathleen Miller, an FDA intern and health economics doctoral student at the University of North Carolina; Clark Nardinelli, chief economist; and CDER Director Janet Woodcock.
Typically, assessment of pharmaceutical industry innovation has centered on the number of new molecular entities and novel therapeutic biologics approved by FDA each year. But the fall off from the peak years of 1996 (49) and 1997 (40) led to concerns about an “innovation crisis,” prompting pleas to Congress to remove regulatory roadblocks to drug approval and institute programs to encourage development of innovative products – though even by NME count, industry appears to be in the third year of a recovery from the sluggish decade of the 2000s (Also see "Encore? FDA Could Come Close To Record 2012 NME Count This Year" - Pink Sheet, 29 Jul, 2013.).
The recent round of PDUFA reauthorization brought in a series of measures intended to encourage and facilitate novel drug development, including moves to encourage broader use of the accelerated approval program, additional exclusivity for antibiotics that treat drug-resistant pathogens and the new “breakthrough” designation program for therapies that show a dramatic therapeutic advantage over existing treatments .
To measure the effectiveness of those programs, the FDA researchers suggest use of a metric beyond the overall NME approval number. While each NME is chemically unique, that does not mean each one provides “a major breakthrough in technology or treatment,” the analysis notes.
Metric For Innovative Drugs
As an alternative, the article![]() suggests measuring approvals within three categories of drugs defined by “their degree of novelty compared to the existing base of pharmaceuticals.”
suggests measuring approvals within three categories of drugs defined by “their degree of novelty compared to the existing base of pharmaceuticals.”
These groups are first-in-class, advance-in-class and addition-to-class. Products that are first-in-class are considered the most innovative because “each represents a new pathway for treating a disease.”
Drugs selected for the advance-in-class group are not first-in-class but were assigned priority review. While they don’t have the novelty of first-in-class, the acceptance for priority review shows that “the candidate therapy can differentiate itself from existing treatments in a clinically meaningful way,” the article states.
The addition-to-class, or “me-too” drugs, “generally represent a lower degree of innovation because at the time of FDA review, they did not distinguish themselves in terms of potential clinical benefit.”
Assessment of 645 drugs approved from 1987 through 2011 using the three categories determined that the decline in approval of new molecular entities since the mid-1990s was driven by industry producing fewer me-too drugs, rather than fewer innovative products (Also see "FDA Questions Innovation Gap, Says Me-Too Drugs Drive Approval Fluctuations" - Pink Sheet, 6 Aug, 2013.).
“When we categorize new drug approvals in a way that recognizes important differences in the novel contribution of a drug, we find that the pace of approvals of pioneering first-in-class drugs has remained relatively stable over time,” while the number of addition-to-class drug approvals has been subject to more fluctuation, the analysts note.
The average for first-in-class drug approvals was roughly eight per year until an appreciable decline to four in 2008 and three in 2009, the article says, noting that the numbers rebounded to nine in 2010 and 12 in 2011 (Also see "Innovation Pays: Priority Drugs Drove Novel 2011 Approvals To New Heights" - Pink Sheet, 9 Jan, 2012.).
They also found a recent trend toward greater sponsor focus on innovative drugs. First-in-class drugs made up 39% of total approvals during 2002-2011, up from 27% of total approvals during the preceding 15 years.
NME Approvals By Innovation Category, 1987-2011
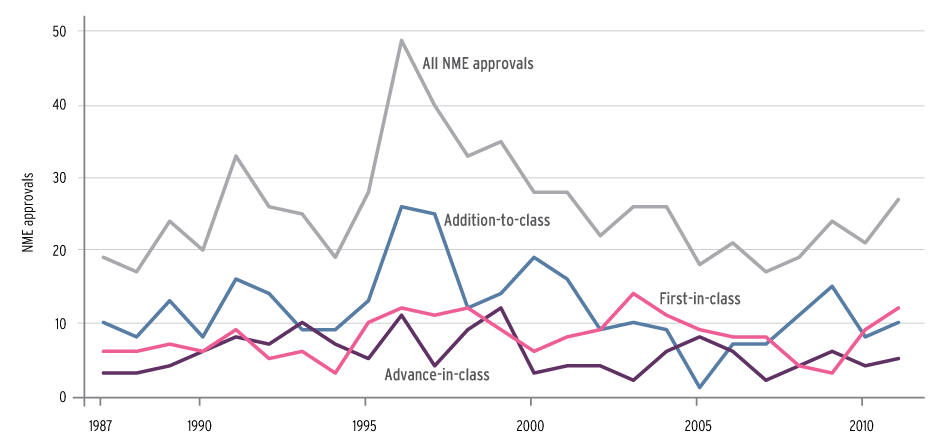
Source: Courtesy of Health Affairs, based on FDA drug approval data
The new statutory and regulatory efforts will continue to push drug developers in this direction and “the sophisticated measurement of innovation will be imperative in tracking the progress being made,” the FDA researchers suggest. “Thus, our observations may be of interest to policy makers as a baseline and method against which the success of these initiatives can be measured.”
Small Firms Catch Up With Big Pharma
The analysis also looks at the spread of approvals between large and small companies, finding that since 1987, large and small firms have produced first-in-class drug approvals in similar numbers. “Both segments of the industry have averaged roughly four first-in-class drug approvals per year, with no substantial upticks or decreases in first-in-class approvals over time.”
A look at approval trends before and after the peak years of 1996 and 1997 found that during the prior nine-year period, the majority of new drugs from large pharmaceutical companies were in the me-too group, while those from small firms were more likely to be first-in-class or advance-in-class. FDA defined large companies as the top 25 in U.S. sales revenue in a specific year based on IMS Health Inc. data, and small companies as those not in the top 25.
From 1987-1995, large firms received 140 approvals and small firms 71. But both segments of the industry produced roughly the same number of first-in-class drugs, 30 for large firms and 28 for small companies.
Large Company NME Approvals By Innovation Category, 1987-2011
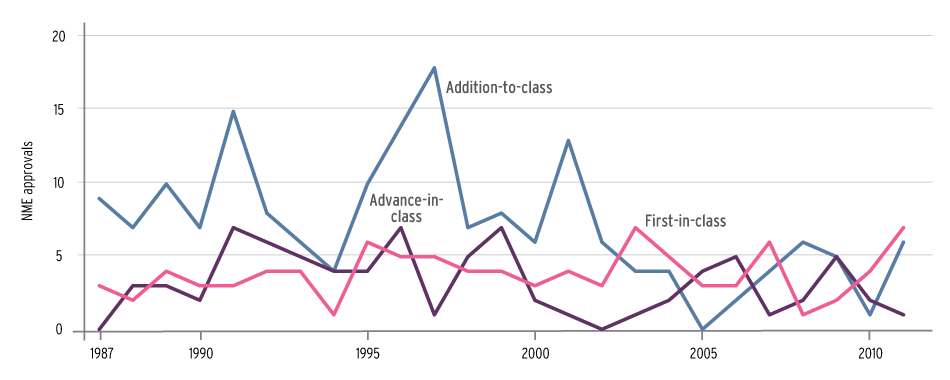
Source: Courtesy of Health Affairs, based on FDA drug approval data
The distribution of approvals began to change in 1996, the article says. In that peak approval year, small companies accounted for 23 approvals, largely due to addition-to-class drugs, and contributed at a higher rate thereafter.
Between 1996 and 2011, small companies sponsored 50% of NME approvals, compared to about one-third of approvals between 1987 and 1995.
Overall, the analysis found that 45% of approvals were obtained by small companies and 55% by large firms. Within the subcategories, however, small firms developed 53% of the first-in-class drugs and 40% of the addition-to-class drugs.
Small Company NME Approvals By Innovation Category, 1987-2011

Source: Courtesy of Health Affairs, based on FDA drug-approval data
Of the 645 drugs studied, 32% (203) were first-in-class, 22% (143) were advance-in-class and 46% (299) were addition-to-class.
The analysis looked at novel drugs and therapeutic biologics approved by CDER in the 25-year period of 1987 through 2011, excluding diagnostic drugs, those approved under the 505(b)(2) abbreviated regulatory pathway and those intended for use solely by U.S. military personnel.
The 645 were assigned to the three categories based on drug class, date of approval and priority review determination. Placement in a drug class was determined by pharmacologic class, the approved indication and specific molecular targets.