How to run clinical trials in the Middle East - why the GCC?
This article was originally published in Scrip
A grouping of Middle Eastern countries came together under the auspices of the Gulf Co-operation Council in 1991. Its six members are Bahrain, Kuwait, Qatar, Oman, Saudi Arabia and the United Arab Emirates (UAE). The GCC countries co-operate in the field of healthcare, among others. The GCC market was valued by BioPlan at around $4 billion in 2008, with major growth expected.
In general, CROs and clinical trial sponsors are drawn to the GCC by:
- patient diversity/genetic variety/patient pools;
- world-class medical structure;
- English competency fostered by US/UK presence (ie, less need for translation);
- lower costs, rapid patient recruitment, shorter timelines;
- regulatory guidelines and government policies aligned with global standards (selected GCC states); and
- favourable logistics.
| |
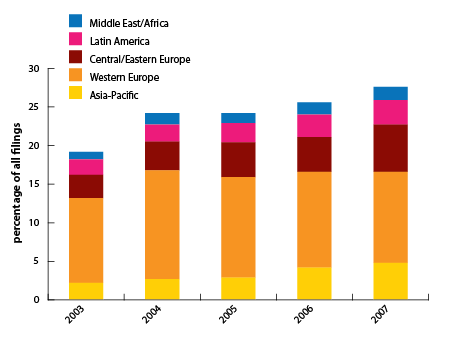
| Figure 1. FDA form 1572s filed by applicants outside North America |
Disease patterns are also considered advantageous. The main causes of mortality are cancer, cardiovascular disease and diabetes. Furthermore, across the Middle East (of which the GCC countries form a major part), there are more than 900 genetic diseases. There is a high prevalence of obesity, with four of the GCC countries ranking in the world's top 20 for this condition. Diabetes is also common, with the latest data suggesting that the UAE has the second-highest incidence of the disease in the world, with more than 20% of the population reported to be diabetic.
Health spending as a percentage of GDP is only 3.1%, well below the OECD average of 8.9%. However, the GCC countries are relatively oil- and gas-rich, so the amount of money invested in health is proportionately high.
By 2025, the number of hospital beds across GCC countries is set to double, with an extra 162,000 needed. GCC countries are investing some $10 billion to make this happen.
Approaches towards drug importation in the region are not clear-cut. Some states will require a licence from the ministry of health. There are also restrictions on clinical studies for products that have not yet been granted approval by Europe's EMA or the US FDA. There are restrictions on early-Phase II and Phase III trials. However, oncology and other specialist products are excluded. Furthermore, some research centres are permitted to waive restrictions (this much be checked by the CRO in advance).
Intellectual property (IP) laws vary across the region, but there is a sense that governments and the legal community are keen to develop them. This would allow at least some protection for innovative ideas/products. There are no hard figures on foreign direct investment. The strengthening of IP rights in the GCC countries, however, will no doubt encourage more of it.
issues to look out for
Although the GCC region is well developed by Middle Eastern standards, there are a number of issues for CROs/sponsors to look out for, including:
- inadequate/inaccurate medical records;
- ethical/legal/cultural considerations for access to medical records;
- informed consent;
- limited storage space and use of filing;
- payment and contract transparency;
- readiness for inspections and audits; and
- drug accountability.
There are also certain cultural differences that could impact on GCP, including:
- the role of physicians;
- healthcare provision by non-physicians;
- infrastructure;
- clinical practice;
- government;
- transportation;
- social hierarchy/gender;
- diet;
- living conditions;
- language; and
- literacy.