Moderna Lauds Joint Work With Health Authorities On Spikevax Safety Monitoring
Executive Summary
A senior executive at Moderna recounts how the company used a variety of data sources to identify potential safety signals for its Spikevax COVID-19 vaccine during a state of “hyper-pharmacovigilance.”
It is no secret that the safety monitoring of COVID-19 vaccines put the pharmacovigilance systems of all sponsors under pressure due to the sheer volume of data, mass vaccination campaigns and high public attention, but it also presented new opportunities to forge collaborative partnerships.
One such partnership that worked for the benefit of public health was between health authorities and industry on signal detection for COVID-19 vaccines, which involves looking at adverse reaction data for patterns to identify new safety risks.
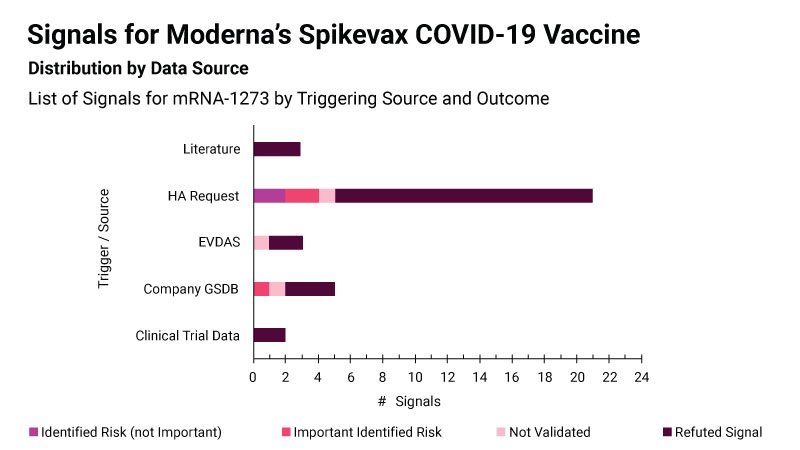
For its Spikevax COVID-19 vaccine, Moderna says it looked at a variety of data sources to identify new safety concerns, but the majority of the signals were triggered by health authorities requesting further assessment of specific concerns in response to their own signal detection activities. (See image)
Most signals for Spikevax were triggered by requests from health authorities Moderna/DSRU
The signal detection work undertaken by health authorities acted as an “additional safety net during the pandemic” and “really helped Moderna,” said Margot Stam Moraga, director for signal management at the company.
Several health authorities undertook such work, including the European Medicines Agency, the UK’s Medicines and Healthcare products Regulatory Agency, Australia’s Therapeutic Goods Administration, Health Canada, Swissmedic and Japan’s Pharmaceuticals and Medical Devices Agency.
The transparent interactions between COVID-19 vaccine marketing authorization holders (MAHs) and regulators on signal detection was a “true partnership” that helped safeguard patient safety. It “was more than regulators telling what MAHs should do – it was a mutual partnership,” Stam Moraga said at a webinar on signal detection organized by the UK-based Drug Safety Research Unit in June.
As for Moderna’s own signal detection strategy for Spikevax, Stam Moraga said that among other things this was shaped by the pre-defined list of adverse events of special interest (AESIs) that required enhanced monitoring, the safety concerns identified in the vaccine’s EU risk management plan, and “health authority commitments which were changing every month.”
Moderna analyzed these aspects using internal, external and public data sources that were reviewed at different frequencies.
Internal Database
The company’s safety database was reviewed every week. Here, the volume of cases was a challenge initially, and a focused review was required. Also, the quality of data in individual case safety reports (ICSRs) was poor.
“Often cases that we received only contained the four minimum criteria to qualify for an ICSR - an event, a product, a subject and reporter,” noted Stam Moraga. To undertake meaningful assessments, the company requested follow-up information and used targeted questionnaires covering specific topics that were already of concern to prioritize follow-ups.
External Databases
The company looked at the EudraVigilance Data Analysis System (EVDAS) every month. This database provides disproportionality analysis, but its main limitation is that it compares the safety reporting for a vaccine versus all medicinal products authorized in the European Economic Area (EEA), explained Stam Moraga.
This means there is a “very high percentage of false flags for review because vaccines and drugs have very different safety reporting patterns,” she noted.
Also, the company looked at the Vaccine Adverse Event Reporting System (VARES) once every two weeks. VARES contains adverse events for all US licenced vaccines and allows sponsors to restrict background data (eg, to focus only on adults when vaccines were initially rolled out in this age group). Also, it can better detect a vaccine-related rare event than EVDAS, the Moderna executive noted.
However, the quality of data in VARES is “inconsistent” because there is a high proportion from patients who only list symptoms, she noted. In contrast, reports by healthcare professionals are more detailed.
Public Data Sources
Moderna undertook weekly literature screening, mainly to identify index cases, class effects, etc. This was “especially helpful because the quality of data from spontaneous reports was quite bad” so “literature ICSRs were very useful,” said Stam Moraga, adding that here also the volume of data was enormous.
It conducted weekly reviews of health authority websites, and tracked labeling updates made to competitor COVID-19 vaccines to keep abreast of class effects. Social medial sites were reviewed monthly using an artificial intelligence tool but yielded very few valid ICSRs.
The company also conducted observed-to-expected analyses every month, which can help refine potential safety signals. Moderna used these to help decide which AESIs it should analyze in more detail.
Also, all COVID-19 vaccine sponsors faced increased regulatory requirements to not just review but also report safety data more frequently. “It was a state of hyper-pharmacovigilance,” Stam Moraga observed. (Also see "EU Mandates Monthly Safety Summaries For Approved COVID-19 Vaccines" - Pink Sheet, 16 Nov, 2020.)
The signal detection activity resulted in some signals being classified as “risks,” which were added to the company’s core datasheet. These included anaphylaxis, myocarditis and pericarditis, dizziness, urticaria, paresthesia and hypoesthesia.
Reflecting on Moderna’s experience with Spikevax’s safety monitoring, Stam Moraga said while the pandemic put pressure on pharmacovigilance systems, it helped the company to streamline its processes and make them robust.
Her advice on dealing with high-pressure safety monitoring is to keep communications simple. For COVID-19 vaccines, “many signals were identified, label changes occurred very rapidly, a lot of health authority requests [were made to review safety concerns] … [so] the simpler you can keep your communication channels, the better.” As “Moderna is a young company, we had more flexibility, but the fast pace was a challenge,” she noted.