Robust Trial Transparency Strategy Boosts Patient Engagement
Executive Summary
Most clinical trial sponsors view data disclosure as a mandate, maintaining regulatory compliance as a legal requirement. Study sponsors that realize the potential to repurpose this data, using a patient-first approach, turn transparency into a strategic advantage for engagement and recruitment efforts.
Sponsor programs to comply with clinical trial disclosure regulations can form the backbone of much broader strategic initiatives for patient engagement – potentially boosting trial recruitment and enhancing information for trial participants.
Expectations for early and comprehensive disclosure of clinical trial data have only been heightened by the global pandemic. Governments and regulators depend on data to refine their COVID-19 strategies, health care providers to assess potential treatment options, and the public to find information and opportunities to participate in research. The pandemic has underscored that posting protocols and clinical results on trial registries in compliance with regulations is a necessary first step start but merely meeting minimum registration requirements does little to communicate meaningful information and reap the benefits of a comprehensive and generous transparency strategy.
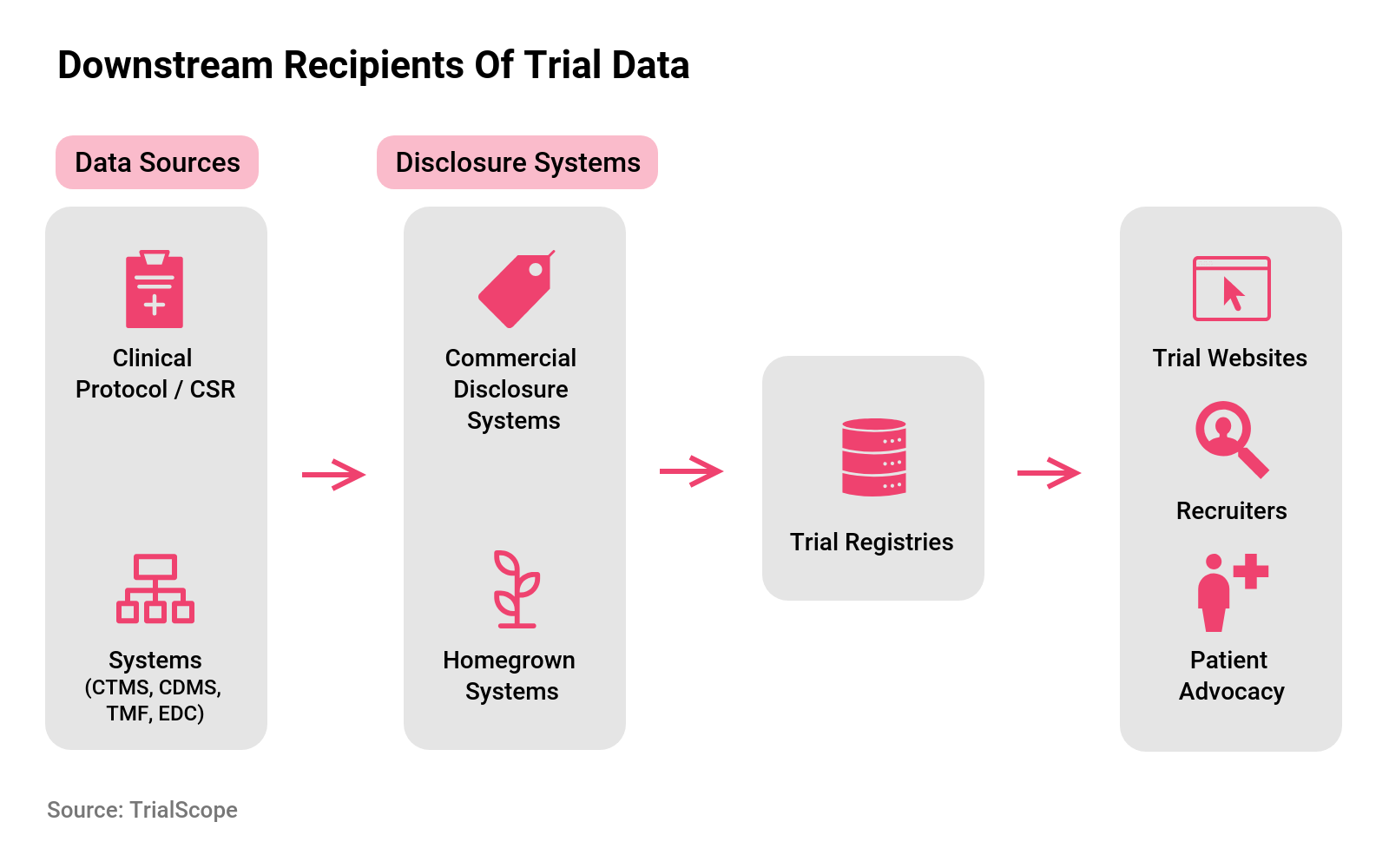
Trial sponsors currently may be reporting data to many of the more than 40 study registries to meet regulatory requirements. The same information can be repackaged on patient-oriented websites, supplemented with educational information about medical conditions, clinical research, and joining a clinical trial which are not available on the registries since these are not designed primarily for the patient.
Explore More On Clinical Trial Transparency
This is the fourth article in a Pink Sheet series on clinical trial transparency strategy and opportunities. See the three boxes further below to delve into our previous coverage.
Also, Thomas Wicks will discuss "Clinical Trial Disclosure As A Strategic Advantage" in a 22 April session of the Informa Pharma Intelligence clinical trials webinar series. Registration is free to participate in the live session or access it on demand.
A robust transparency program can support the trial-finder websites hosted by many biopharma companies, which list their trials in patient-friendly format and content. Some organizations also provide trial-specific web pages informing prospective participants about the research, enrollment criteria, study locations, and related materials to aid with recruiting. The information is also accessed by recruiters and by patient advocacy groups to inform their members.
Those taking this more holistic approach can also see a ripple effect from specific elements such as expanded use of plain language in trial information and other measures to improve clarity in communication. The plain protocol information, for example, is repurposed in the informed consent forms and patient engagement initiatives made publicly available on registries and sponsors’ trial finder websites. Upon completion of the trial, these protocol elements form the core of the plain language summary (PLS) of results that will be mandatory for all trials conducted in the EU starting in 2022. Many sponsors are already providing PLS to their trial participants.
Consistency, Clarity Are Key
Submitting data to clinical trial registries should be considered an integral part of the communications continuum rather than a stand-alone exercise to satisfy regulatory requirements. Trial information is disseminated through regulatory submissions, posters, abstracts, journal articles, investigator brochures, informed consent forms, and corporate and investor communications. A coordinated approach is required to ensure that wherever trial information is available, it is consistent, clear, and, of course, accurate. While consistency is perhaps the most difficult of these three to achieve as the data are made available at different times and for different purposes, clarity is all too often given short shrift, especially when disclosing on clinical trial registries.
Although no global data disclosure standard exists for data reported from trials conducted in multiple countries and reported in multiple registries, most registries require a common set of information based on the WHO Trial Registration Data Set. To the degree allowable by the individual trial registry requirements, the information for these elements should be the same on all registries. This is especially true when listing the primary and secondary outcomes, where difference can create the impression of selective disclosure.
Putting the patient’s information needs at the center of this ecosystem is key to maximizing the value of the time and effort put into disclosure and transparency. Realizing that the purpose of disclosure is not merely to satisfy regulatory compliance, but to support patient engagement and recruiting, points to the importance of clear and consistent posting of clinical data across all registries and websites.
Disclosure best practices include establishing a central function to track and manage global protocol and results registration. This central coordination includes preparing a standard set of data approved for posting to local registries by affiliates, partners, and CROs. The standard data includes, at a minimum, the elements of the WHO Trial Registration Data Set but is often based on the information submitted to ClincialTrials.gov or the EudraCT. The standard disclosure data set, reviewed and approved by a central stakeholder group, also will:
- significantly reduce the local disclosure effort and editorial decisions to streamline global processes,
- avoid the inadvertent sharing of information that could jeopardize the ability to file a patent, and
- eliminate later challenges to redaction of company confidential information if the data were made publicly available by one of the regional registries.
To improve the clarity of publicly available information and further improve global consistency, sponsors are increasingly anticipating the downstream disclosure requirements in source documents such as the protocol and clinical study report (CSR). Authoring with disclosure in mind means incorporating plain (lay) language elements to the protocol and the CSR synopsis, and considering the structure of the documents to facilitate eventual redaction, such as moving patient narratives to an appendix in the CSR.
While protocols and CSRs are clinical documents designed to communicate scientific and medical concepts to health authorities and appropriately trained researchers, certain sections can be presented in plain language. For example, the protocol title is intended for a professional audience, while the brief title can be written in language that someone without a scientific background readily understands. Similarly, the brief description, key eligibility criteria and even alternative versions of the primary and secondary endpoint descriptions may be presented in simple language.
Consistency and clarity of information also build trust in the sponsor among the public, investors, and the health care community. Conversely, inconsistent and confusing data can damage a sponsor’s brand reputation, translating into lack of confidence for its products, partnerships, and investment potential.
Article 35 of the Declaration of Helsinki states that “Every research study involving human subjects must be registered in a publicly accessible database before recruitment of the first subject.” Organizations that refer to the declaration in their ethical principles are committing to the public registration of all trials in humans, including early Phase I trials, even if that commitment does not always translate into action. Nonetheless, trial sponsors increasingly agree to register all interventional trials and disclose all results.
A comprehensive disclosure policy that goes beyond mere compliance with regulations reveals a strong organizational commitment to transparency. The expanded public availability of trial data is recognized in assessments such as the FDAAA Trials Tracker and EU Trials Tracker by the DataLab at the University of Oxford, regular publications by TranspariMED, and in the Good Pharma Scorecard by Bioethics International.
In addition to the positive impact on the organization’s reputation and image, the public disclosure of all trials, posted with up-to-date, clear, and globally consistent information, supports patient engagement initiatives and accelerates recruitment.
However the information is accessed, it must be relevant, current, and harmonized across all outlets. The good news is that once the commitment has been made to coordinate disclosure globally, the effort to prepare a standardized data set for dissemination eliminates duplicative editorial processes for each outlet while improving the publicly available data’s consistency and value.
Thomas Wicks is chief strategy officer at TrialScope. He has more than 20 years of experience with performance and content management approaches in the life sciences, with particular focus on clinical trial transparency.
.jpg?rev=302b1310147f4c219c8586e69fce802e&h=141&w=120&la=en&hash=A4CA4649E83F30DFDE0CB28209DEF1E5)