Amazon OTCs Likely A Worry For Retailers, A Remedy For Perrigo
Executive Summary
Amazon introduced Basic Care line of cough, cold, allergy and digestive treatment OTCs developed in a partnership with Perrigo. The range could be a major threat to store brand and private label OTCs, though success may depend on whether consumers wait for Amazon deliveries or shop at stores.
[Amazon.com Inc.]’s Basic Care OTC drug line might become a "comprehensive threat” to retailers' brands, says a health care market analyst, but there's little doubt about its benefit to manufacturer Perrigo Co. PLC.
Raymond James Financial Inc. analyst John Ransom recently noted Amazon's quiet Basic Care launch and cautioned other retailers about the new competition. “While we aren’t dialing up our scientific, proprietary ‘sky is falling freakout-o-meter’ to 11, we do think that this move bears monitoring,” Ransom says.
Basic Care OTCs include Anti-Diarrheal Loperamide HCL Tablets, Nicotine Gum (nicotine polacrilex), All Day Allergy (cetirizine hydrochloride 10 mg), Allergy Relief Loratadine tablets (10 mg), Ibuprofen (200 mg), Acetaminophen (500 mg), Extra Strength Acetaminophen PM caplets (acetaminophen 500 mg, diphenhydramine HCL) and Naproxen Sodium tablets (200 mg).
Children’s products include Pain & Fever (acetaminophen 160 mg) and ibuprofen 100 mg.
Product packaging compares each item to a national brand, such as Basic Care Anti-Diarrheal product packaging states “Compare to Imodium-AD,” and All Day Allergy packaging states “Compare to Zyrtec.”

Labeling for Amazon's Basic Care OTCs, such as its loratadine allergy treatment, compares each item to a national brand.
The products, available on Amazon since November, are less expensive overall and in some cases “much cheaper” than similar private label products offered by retailers, Ransom said in a Dec. 22 brief.
Basic Care ibuprofen 200 mg in a 500-count package retails for $7.50, while CVS’ ibuprofen in the same dosage and count retails for $11.99 and Walgreens Boots Alliance's for $15.49. Basic Care’s Omeprazole 20 mg product is priced at $15.99 for a 42-count offering; CVS’s similar product costs $21.99 and Walgreen's $21.99.
Success Swings On Consumer Habits
“We believe Amazon rolling out private label generic OTC further expands Amazon’s strategy of offering a wide selection and lower prices to consumers,” says Ransom.
Still, taking market share from other store and private-label brands depends on consumers turning from relying on conventional stores for purchasing drugs as soon as they experience illness symptoms to buying OTC products from online retailers independent of immediate need.
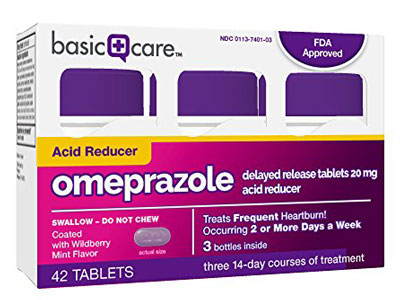
Amazon prices its OTCs below other private label lines, such as Basic Care Omeprazole 20 mg, 42-count, at $15.99; CVS’s and Walgreen's similar products are priced at $21.99.
“Currently, 65% of OTC market is analgesics, antacids and cough/cold, and we don’t believe customers stock these items in advance vs. purchasing them spontaneously when the need arises,” Ransom says.
Perrigo CEO John Hendrickson shared similar sentiments about Amazon's OTC potential and consumers' purchase habits at September investor conferences, projecting that until the e-commerce giant could deliver OTC drugs in a few rather than in 24 hours, most consumers would continue to rely on local stores. Hendrickson also said Perrigo would work with Amazon should it ask. (Also see "Perrigo Positioned To Prosper From Potential Amazon OTC Product Play" - Pink Sheet, 20 Sep, 2017.).
Nonetheless, Ransom says Amazon’s private label OTC entry could take share not only from higher price items in the OTC drug segment, but also in chronic disease treatment categories, such as smoking cessation, “where purchase can be planned and timed.”
Analysts have speculated for some time that Amazon would launch its own OTC line and would tap Perrigo, the leading supplier of private label OTC drugs, to manufacture the products. Perrigo, which maintains its primary office in Allegan, Mich., provides 4,000 formulas across 7,300 stock-keeping units and has most major US retailers among its customers. It also markets its own GoodSense OTC drug brand, available in discount stores and on Amazon.
$1M In Amazon Supplement Sales
Amazon markets around two dozen brands that span a range of CPG categories, from food and beverages to hardware, fashion and personal care. Online market researcher OneClick reported Amazon’s brands showed “record” growth in 2017, up 10% from 2016 to $400m, with the bulk of sales in its AmazonBasics line of home tech accessories, batteries and office supplies.
Amazon Elements vitamins and supplements launched in early 2017 and had sales of around $1m, OneClick says. Amazon Elements baby wipes nearly doubled its 2016 estimated sales with 94% growth to $15m in 2017.
Ransom said Amazon’s brands account for around 2% of its total business, though “overall, we expect private label will be an increasing focus for Amazon going forward.”
Sales of consumer health and personal care brands online and in stores have been battered over the past year from lower-priced private label products sold exclusively online, particularly on Amazon. (Also see "Pricing Pressures 'Manageable' To P&G, But Threatening To Analysts" - Pink Sheet, 17 Nov, 2017.)
Basic Care and GoodSense products are among the top results for OTC drug ingredients in Amazon searches, and the firm also pairs an “Amazon’s Choice” designation with many GoodSense products. For example, a search for “ibuprofen” results in a Basic Care and GoodSense product in the top six results, with the “Amazon’s Choice” attached to the latter product.
Products' placement in Amazon search results can be critical for a brand, given that the e-commerce platform is the leading source for product research: 55% of consumers using the website research consumer packaged goods items. (Also see "Navigating Amazon: E-Commerce Giant Critical For OTC Drug Sales" - Pink Sheet, 4 May, 2017.) Amazon says it uses an algorithm for search results that favors better-selling products with high customer satisfaction ratings, but also incorporates price and availability.
Perrigo also became a more enticing partner after investment fund Starboard Value LP upped its stake in Perrigo almost 2 percentage points to 6.5% in September and declared Perrigo the “best idea now” for pharma industry investment. Starboard also noted Perrigo would prosper from a potential Amazon pharma play. (Also see "Perrigo Glows From Mucinex Private Label Plan, Starboard Plug" - Pink Sheet, 15 Sep, 2017.)
Amazon Part Of Perrigo's Online Turn
Perrigo spokesman Bradley Joseph would not provide specifics on the development of Basic Care but said the firm has been working with Amazon to develop its own brand of OTCs with labeling and packaging designed to incorporate “call-outs that are most important to consumers to better support their own OTC product purchase decisions while making the specific own label comparison to the equivalent national brand clear and concise.”
Joseph, vice president global investor relations and corporate communications, noted the importance of online brands to Perrigo's continued growth. "Through [its] partnerships with retail customers, Perrigo is broadening its experience in marketing consumer healthcare products online," he said in an email.
"As part of this initiative, Perrigo has been working with Amazon to develop an own brand line of OTC products under a label that would be exclusive to Amazon," Joseph said.
Dublin-based Perrigo filed the trademark for the brand in March 2017, according to US Patent and Trademark Office records. The brand covers allergy relief medications, analgesics, antacids, antihistamines, antitussive cold preparations, anti-diarrheal preparations, cough syrups, expectorants, decongestant capsules and tablets, decongestant nasal spray, laxatives, medicinal gastrointestinal preps, pharmaceutical preps for the treatment of flatulence, sleeping tablets, medicated smoking cessation gums and lozenges.
Ransom says it's too early to gauge the impact of Amazon's OTC line on sales of Perrigo’s brand and the store brand and private label products it provides to retailers.
“We don’t view Amazon’s entry or increased presence in the OTC channel as an incremental win or loss at this early stage, but rather a reallocation of market share from traditional to online. Regarding price points, we believe Perrigo sells to Amazon at the same price as the retail chains and therefore it doesn’t really matter where the business goes.”
Although it remains the dominant OTC store brand and private label provider with around 65% US market share, Perrigo earnings faltered and its share price dropped substantially over most of the previous two years after it fought off Mylan NV's hostile takeover bid, spent $4.5bn to acquire Omega Pharma NV as a platform to start marketing brand and private label consumer health products in Europe and weathered shrinking prices for the generic topical specialty drugs it manufactures. (Also see "Perrigo's Return To OTC Roots Restoring Investor Confidence" - Pink Sheet, 1 May, 2017.)
Basic Care Children’s OTCs include ibuprofen 100 mg.
Perrigo shares were trading near $200 when Mylan announced its bid in April 2015, and the share price varied from that level to around $180 through mid-September that year, when the shares began a steady slide that reached $146.90 the day Mylan's tender failed. The share price largely has fallen since, including a low for the previous six month of $66.37 in mid-August and a high of $89.90 in late December.
Perrigo shares got a slight boost on Jan. 2 following the firm's announcement of FDA's tentative approval of its abbreviated new drug application for generic bromfenac ophthalmic solution 0.07%, a nonsteroidal anti-inflammatory drug indicated for postoperative inflammation and reduction of ocular pain. The price reached $90.71 from a Dec. 29 close of $87.16, and the boost sustained Jan. 3, closing at $89.44.
The potential revenues from providing Amazon's OTC line could be a tonic for much of what has ailed the firm.
In September, Hendrickson said he expects an Amazon OTC play to help grow the total category rather than slice up current competitors' market shares as more consumers would buy OTC products online, looking for the best offers just as they do in stores.
In a Jan. 3 research note, Deutsch Bank analyst Gregg Gilbert said the Basic Care launch is another in "the early innings of the on-line sales opportunity for OTC products," which bodes well for Perrigo as the manufacturer "well-positioned within this evolution."
The near- and medium-term potential for Perrigo from Basic Care sales is not impressive, "but the longer-term opportunity would be tied to higher rates of conversion from national brands to store brands (a market that [Perrigo] dominates)," Gilbert said.
He noted, though, that Perrigo is working with Amazon while it remains the dominant provider of the same OTCs for conventional retailers. Just as Amazon was mum as Basic Care sales began, Perrigo has not promoted its work with the firm.
"We would not expect [Perrigo] to 'play up' this development as Amazon is for now just a small customer and [Perrigo] would not want to rock the boat unnecessarily with its larger customers," Gilbert said.
Perrigo in November reported overall third-quarter net sales down 2% to $1.23bn, net income of $45m, or 31 cents per share, after reporting a net loss of $1.59bn, $11.10 per diluted share, in the prior-year period. (Also see "Private Label Nexium OTC Buoys Perrigo As Pricing Trims Rx Revenues" - Pink Sheet, 10 Nov, 2017.)
A launch of private label OTC Nexium24HR (esomeprazole 20 mg delayed-release capsules) late in the quarter was enough to help drive $13m in new consumer health product sales in North America but not enough to pull the division out of a 2% dip. Reported North American OTC drug and adult and infant formula sales during the July-September period were $599m as the firm lost $21m in sales from divesting its vitamin, mineral and supplement sales division, according to its Nov. 9 earnings release.
Its international consumer business, operating primarily in Europe and in Australia and Israel, reported a 3% drop in net sales to $365m on a 6% loss to currency exchange rates and $42m lost from discontinued operations.
While its Rx topical specialty products business reported net sales of $251m, down 1% on losses to anticipated pricing pressures, Hendrickson said rather than divesting the business as Starboard has recommended, Perrigo expects the sector to grow.
It's likely, though, that Hendrickson's confidence in remaining in the Rx generic topical business influenced his announcement in June 2017, after little more than a year after taking the helm, that he would retire upon the appointment of a successor. The decision came a month after Starboard built a five-seat bloc on Perrigo's board. (Also see "Perrigo CEO Takes Faith In Rx Generics Business To Exit Door With Him" - Pink Sheet, 6 Jun, 2017.)
From the editors of the Tan Sheet.
