Could adaptive designs be the answer to oncology clinical development success?
Executive Summary
Across all therapeutic areas, clinical development faces well-documented, critical challenges that impact the pharmaceutical industry's ability to bring new medicines to patients – but in the oncology space, these issues are particularly acute. New strategies are urgently needed to help improve the probability of clinical trial success in oncology. In this article, we examine adaptive trial designs, recently championed as a promising approach by US Food and Drug Administration (FDA) Commissioner Scott Gottlieb in his confirmation testimony to the US Congress [1] Adaptive trial designs can help address the challenges encountered in anti-cancer clinical development today by saving time, resources and improving the odds of success.
[2]
The continued challenges in oncology clinical development
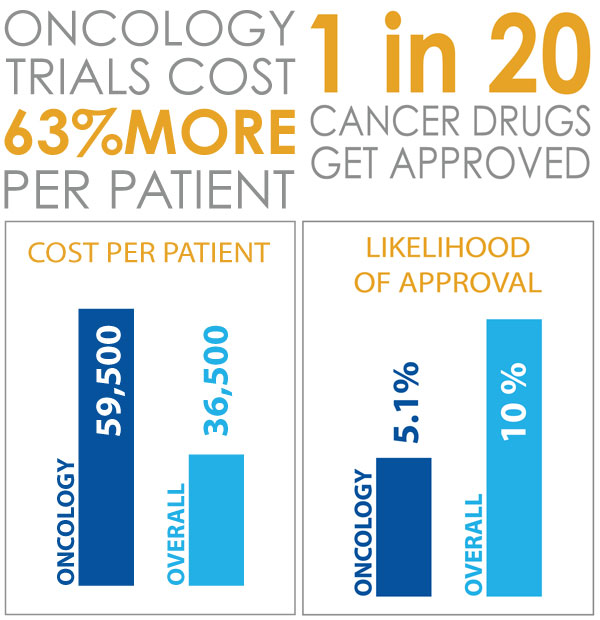
Sadly, clinical development of anti-cancer therapeutics faces particularly high rates of failure, even in the context of low success in drug development as a whole. A 2016 BioMedTracker report [3] reviewed nearly 10,000 clinical and regulatory phase transitions between 2006 and 2015 to calculate the likelihood of approval from Phase 1. Out of 14 major disease areas that were tracked, oncology had the lowest Likelihood of Approval (LOA) at only 5.1%, compared with an overall LOA across development of just under 10%. Operating costs for oncology trials are also higher than those observed across clinical development as a whole with an estimated cost per patient of $59,500 per patient in oncology, versus $36,500 across all disease areas. [4].
One contributing factor to the particularly high failure rates in oncology is the uncertainty about the treatment effect or expected benefit. In a 2012 article, Gan et al [5] analyzed over 250 published oncology trials. The authors found little correspondence between the expected and observed benefits. More importantly, the expected benefits consistently overestimated the observed benefits often by a large margin.
Another issue is the ability to generate sufficient clinical evidence, due to patient recruitment difficulties. Currently, only 3% of adult cancer patients enroll in clinical trials [6] and this creates pressing challenges for oncology drug developers. It means that data are scarce, and this becomes even more problematic when developing medicines for very rare cancer indications such as Acute Myeloid Leukemia, Chronic Myeloid Leukemia, Angiosarcoma and many other indications, where different drivers of disease lead to distinctions between sub-populations.
Adaptive strategies to improve probability of success
Faced with these challenges, drug developers, regulators, patients and other stakeholders in clinical development are turning to innovative approaches capable of enhancing the traditional development paradigm, and improving the probability of success.
A leading strategy that has risen in popularity in recent years is the adaptive trial design. When used appropriately, adaptive designs may help to mitigate against some of these challenges – maximizing the value of the clinical data generated in a trial to reduce risk, improve efficiency and deliver benefits to both future patients and clinical trial participants.
With a critical need to bring vital new medicines to patients, the oncology research community has been particularly receptive to applying adaptive trials. In fact, according to a recent review article, [7] oncology is the therapeutic area which that has seen the greatest uptake. Regulators have also seen the potential. For example, as part of the Prescription Drug User Fees Act the FDA has committed to a series of activities ‘to facilitate the advancement and use of complex adaptive, Bayesian, and other novel clinical trial designs.’ [8]
So, what are adaptive trials?
While a traditional randomized controlled trial has fixed parameters which are defined at the outset and maintained throughout, an adaptive trial reviews the accruing data from patients at one or more times (called ‘interim looks’) as the trial is on-going. Based on the information obtained at these points, researchers can then make robust, pre-planned changes to the parameters of the trials. [9,10] The central concept behind adaptive trials is simple: to reduce the uncertainty and inherent risks in clinical development by obtaining additional information from the ongoing trial and using it to make better decisions. A Bayesian statistical framework is often particularly well suited to adaptive trials since it naturally allows the totality of evidence to be updated as new data become available.
How can the adaptive trial benefit clinical development and improve the probability of success?
On the basis that richer information is always better, at a high level, the use of adaptive trials can lead to better decisions about what to do during the course of clinical development and ultimately improve the probability of success. More specifically, adaptive trials can deliver commercial and budgetary benefits to biopharmaceutical companies, and ethical advantages to both participants of clinical trials and future patients of desperately needed new medicines.
By using an adaptive approach, more complete information can be obtained on the drug and its dosage, resulting in a much greater chance of the Phase 3 confirmatory trials being successful, with a reduced probability that the Phase 3 dose will either be toxic or show inadequate efficacy. The time-to-market for a drug can also be reduced by combining proof-of-concept trials (to show that the drug works) with dose finding trials (to pick the right dose) or by combining dose-finding trials with confirmatory trials. This eliminates the ‘white space’ between the end of one trial and the start-up of the next. Certain kinds of adaptive approaches can help to reduce the number of patients required in a trial or allow the commitment to a larger number of patients to be made only when there is enough information to know there is an acceptable probability of success. This delivers both budgetary advantages to the pharmaceutical company and ethical advantages to patients.
The participants in the clinical trial stand to benefit from an ethical standpoint, as it is possible to design trials that improve the outcomes of patients within the study. For example, trials can be discontinued when it isn’t likely that the therapy is effective or if the therapy notably improves benefit-risk compared to the control. At a more global level, when researchers are able to make earlier decisions about ineffective drugs, this allows intellectual capital and resources to be redeployed to other development activity. In other words, improving the overall efficiency of the research effort provides an ethical benefit in the drive to bring new medicines to patients. [11]
When in development can adaptive trials be applied?
Adaptive trials are used in both exploratory (early) and confirmatory (later stage) development. The FDA’s draft guidance on adaptive designs [12], which is fairly supportive of their use in development on the whole, is particularly encouraging of their application in the exploratory setting. In exploratory clinical trials, adaptive designs are primarily focused on finding safe and effective doses or with dose–response modeling. In a confirmatory setting, robust, planned changes are made to the future course of an ongoing trial based on analysis of accumulating data from the trial itself. This is intended to improve decision-making. As we would expect, from both a regulatory and operational point of view, there is more complexity involved in applying adaptive approaches to confirmatory trials, to ensure that the validity of the trial is maintained and that any bias is avoided. Nevertheless, benefits are available for those prepared to invest in the upfront planning required.
How are adaptive trials applied in oncology exploratory development?
The primary objective of nearly any Phase 1 clinical trial in oncology is to conduct dose-escalation to identify the Maximum Tolerable Dose (MTD) of a new drug. Administering a dose that exceeds this MTD can cause serious toxicities for a patient who is already ill. However, a dose that falls below the MTD generally fails to offer patients the full benefits of a therapy. As a result, determining the MTD as quickly as possible is normally of paramount concern for a Phase 1 trial.
Traditional approaches to dose escalation are based on a set of rules; an example of this is the commonly used 3+3 method in which three patients are initially enrolled into a given dose cohort. Where no Dose-Limiting Toxicity (DLT) is observed in these subjects, then the trial may proceed to enroll additional subjects into the next higher dose cohort. Such designs are widely used in early clinical development and can be very appealing due to their simplicity. However, they are not necessarily the most successful way of targeting the true MTD.
Innovative model-based approaches to dose escalation early phase trials such as the Bayesian Logistic Regression Model, Modified Toxicity Probability Interval, or Continuous Reassessment Model, can offer an effective alternative to determining the MTD of a new drug. They can also ensure that all of the information available to trial clinicians is taken into account so that the patients enrolled in the trial receive the best possible treatment. In oncology, the ability to design early phase trials to support combination therapies is extremely important, and there are also reliable, flexible dose escalation designs to support dual agent trials, which are now being applied in practice.
With increased familiarity around these strategies, several leading pharmaceutical companies are now incorporating Bayesian and adaptive methods for many of their early phase oncology clinical trials.
How are adaptive approaches applied in oncology confirmatory development?
Adaptive trials can also help to manage the very significant risks of later stage development. There are a number of approaches available to researchers at this stage. Designs that adjust sample size while the trial is ongoing (sample size re-estimation designs) can help sponsors to mitigate the risk of underpowering their study by verifying key assumptions based on interim data. Importantly, when patient recruitment is difficult and costly, they also allow a smaller commitment to be made upfront until more, hopefully positive, information can be obtained from an interim analysis. In oncology, these designs lend themselves best, but not exclusively, to indications where a large number of events is observed quickly, such as Acute Myeloid Leukemia, Metastatic Lung or Colorectal Cancer.
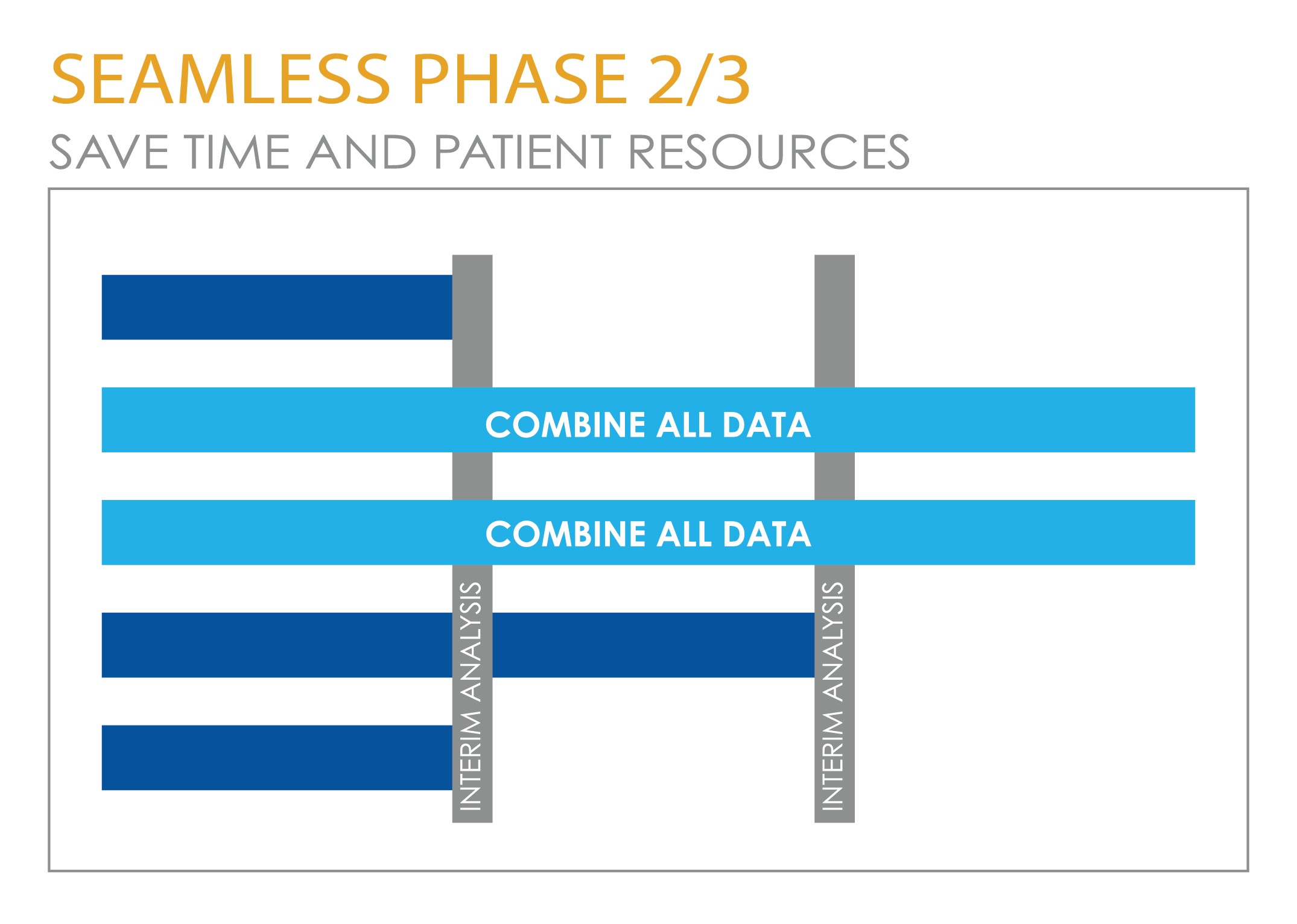
A combined Phase 2/3 or 'seamless' design can also help to save time and patient resources, by removing the white space between phases and supporting an informed go/no-go decision based on early-read endpoints like Objective Response Rate (ORR) or Progression Free Survival (PFS). The decision to pursue the expensive Phase 3 portion of the trial based on the frequently required regulatory endpoint of Overall Survival can then be based on within-trial evidence of a benefit.
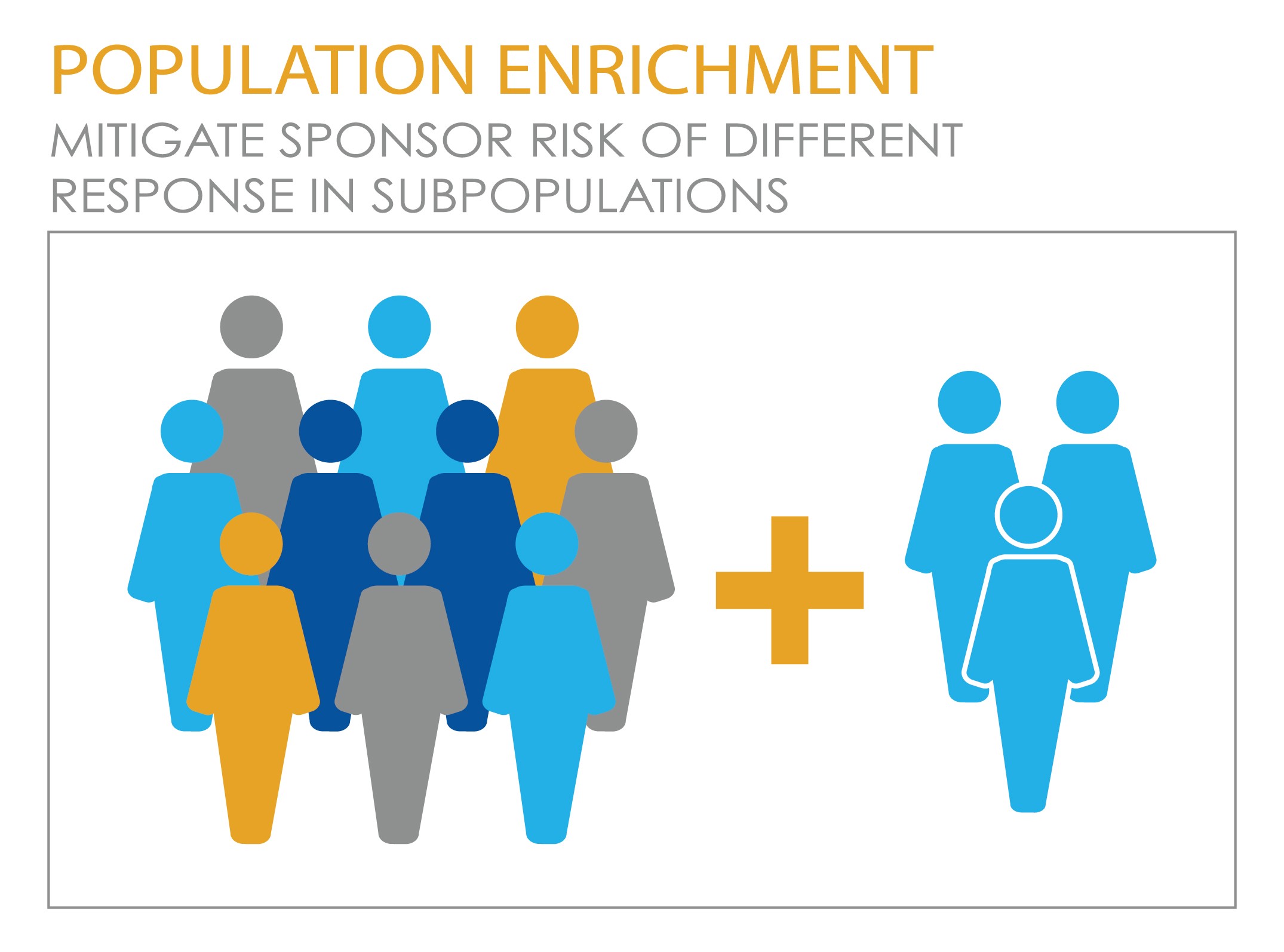
Another adaptive design of mounting interest in the era of personalized medicine is the adaptive population enrichment design, which can mitigate sponsor risk in cases, common in oncology, where there is a potential difference in treatment response between sub-populations. This kind of design essentially adapts the eligibility criteria for the study to allow patients to be recruited entirely from the responsive subgroup if the drug doesn’t show efficacy population as a whole. On the other hand, if it does show efficacy in the whole population, the study is able to proceed accordingly.
It is important to point out that in any adaptive design used in confirmatory trials, one critical consideration is to ensure that ‘Type 1 error’ is strongly controlled. Type 1 error is a statistical concept meaning the incorrect rejection of a null hypothesis and more generally understood as the risk of a ‘false positive’. In other words, the design must show that it does not inadvertently introduce bias and risk an ineffective drug being declared effective.
The future for adaptive trials
Clearly, no trial design - whether adaptive or otherwise - can make an ineffective drug work. However, a well-designed adaptive trial may help maximize a drug’s chances of showing its true benefit if that benefit does indeed exist. Particularly in oncology development, with success rates so low, biopharma companies should aim to conduct a careful evaluation of all design options, including adaptive strategies to decide on the best approach for the individual trial’s circumstances. Of course, since adaptive trials are often more complex to design and execute than fixed trials, companies considering these approaches should also ensure that they have the right level of statistical and operational expertise to support them- whether this is in-house or courtesy of an outsourced provider.
As we see uptake among biopharmaceutical companies rising, an accompanying increase in practical experience, and a commitment from regulators to support their use, adaptive trials are now set to take their place firmly in the mainstream of oncology research, with an important role to play in the collaborative effort to improve outcomes for sponsors and patients.
About Cytel
Cytel is shaping the future of drug development. Our software for design, analysis and execution of clinical trials is used by all leading pharmaceutical, biotech and medical devices companies. As the world's largest Biometrics CRO, we improve our customers’ chances of success through expert trial design and planning, efficient operational implementation and accurate data analysis.
Click below to connect with Cytel’s biostatistics experts and discuss how an adaptive approach could support your oncology development objectives.
About the author
Yannis Jemiai, Ph.D. is Senior Vice President at Cytel, where he leads the software products, strategic consulting, and marketing groups. His research interests include drug development, oncology research, adaptive trial designs, dose-finding studies, predictive analytics, causal inference, and missing data. Dr. Jemiai received his Ph.D. in biostatistics from Harvard University.
References
- Servick, K. (2017). Congress and FDA nominee heap love on ‘adaptive trials’. Science
- American Cancer Society – Cancer Facts and Figures 2017
- Clinical Development Success Rates 2006-2015 Biomedtracker
- Biopharmaceutical Industry-Sponsored Clinical Trials: Impact on State Economies PhRMA/ Battelle
- Gan, H., You, B., Pond, G. and Chen, E. (2012). Assumptions of Expected Benefits in Randomized Phase III Trials Evaluating Systemic Treatments for Cancer. JNCI Journal of the National Cancer Institute, 104(8), pp.590-598
- Transforming Clinical Research in the United States: Challenges and Opportunities: Workshop Summary
- Hatfield, I., Allison, A., Flight, L., Julious, S. and Dimairo, M. (2016). Adaptive designs undertaken in clinical research: a review of registered clinical trials. Trials, 17(1)
- PDUFA Reauthorization Performance Goals and Procedures Fiscal Years 2018 Through 2022
- European Medicines Agency Reflection Paper on Methodological Issues in Confirmatory Clinical Trials Planned with an Adaptive Design
- FDA Guidance for Industry: Adaptive Design Clinical Trials for Drugs and Biologics Draft Guidance
- Legocki, L., Meurer, W., Frederiksen, S., Lewis, R., Durkalski, V., Berry, D., Barsan, W. and Fetters, M. (2015). Clinical trialist perspectives on the ethics of adaptive clinical trials: a mixed-methods analysis. BMC Medical Ethics, 16(1)
- FDA Guidance for Industry : Adaptive Design Clinical Trials for Drugs and Biologics Draft Guidance