'A huge step' towards global biosimilars development - UPDATE
This article was originally published in SRA
Executive Summary
Planned change on the choice of reference product for biosimilars in the EU means global development is on the agenda. Maureen Kenny reports from the EGA biosimilars conference in London.
The EU is preparing for a major change in biosimilars regulation that the generics industry says will open the door to global biosimilars development. Assuming it goes ahead, the change could mean a huge reduction in the number of clinical trials that biosimilars developers wishing to market their products in the EU have to conduct.
The change would allow the European Medicines Agency to rely on data from comparative testing performed against reference product sourced outside the EU. Biosimilar product applicants would therefore no longer have to repeat all clinical studies against reference biological products sourced from the EU.
The proposed approach is in line with that of US Food and Drug Administration draft guidance and, according to the European Generic medicines Association, it could reduce the cost of developing a biosimilar by as much as 100 million Euros. Pending positive feedback from the European Commission, details will be included in the revised draft of the so-called "overarching" EMA guideline on biosimilars, CHMP/437/041-3, which has been in place since 2005. This is expected to be issued for public consultation in the third quarter of this year.
Senior officials from the commission and the EMA – Nils Behrndt and Peter Richardson respectively – announced the likely change at the 10th annual EGA international symposium on biosimilar medicines in London in April4.
The current interpretation of the relevant existing EU legislation – Directive 2001/83/EC (as amended) – is that the reference product should be sourced (or batch released, to use US terminology) in the EU and that data generated with a reference product sourced outside EU can only be considered “supportive”.
However, Mr Richardson said, discussions between the EMA and the commission were ongoing regarding a more flexible interpretation of the directive to allow for comparative testing against a reference product in a country outside the EU. The originator biological reference product would still have to be authorised in the EU and bridging data would normally be required to support the use of a foreign-sourced reference product comparator.
Mr Behrndt for his part said he was “prudently optimistic” that the text of the directive was flexible enough in this regard and that “we just need to amend the relevant guidelines”.
The scientific approach the EMA is proposing is in line with that of the FDA, Mr Richardson noted. Recent draft US guidance5-7 foresees – subject to scientific bridging between reference products – the possibility of using reference product sourced outside US to avoid repetition of clinical trials. "We would like to be able to, to the extent possible, bridge the European data," Rachel Sherman, director of medical policy at the FDA's Center for Drug Evaluation and Research, said when the US guidances were released8. "We would try very hard to make that bridge," she said, acknowledging that Europe is "a number of years ahead of us".
There were some words of caution from Mr Behrndt. While the commission was “keen and willing” to reconsider the current interpretation and so allow sourcing from different regions, it still needed to get “final legal clarity” on the matter and Mr Behrndt couldn’t “promise the result”.
Despite these caveats, the commission official’s remarks were greeted with delight.
Joerg Windisch, Sandoz Pharmaceutuicals’ head of global technical development and a session chair at the symposium, described the development as “a huge step forward” and said that he had had “no expectation that there would be such good news”.
With 19 products already having gone through the marketing authorisation approval process in the EU, the biosimilars industry was “no longer the new kid on the block”, Mr Windisch, who is also chair of the EGA European Biopharmaceuticals Group (EBG), had told the meeting earlier.
“Global development is on the EU agenda. It will happen,” Suzette Kox, the EGA’s senior director scientific affairs, announced in her closing remarks to the symposium. “The biosimilars industry is finally getting the same level playing field as the originator industry,” Ms Kox said, adding that the development should be welcomed by the whole industry “as almost every biologics company will go into this space”.
Indeed, as Ms Kox noted9, an attendee from Pfizer voiced support for global development at the symposium. Also, at last year's symposium, a presenter from Merck voiced similar support, saying that regional comparator requirements posed a major challenge to companies in terms of designing a global biosimilar programme.
“It is also great news in particular for patient access to biopharmaceuticals and the sustainability of the EU healthcare systems,” Ms Kox said.
According to Mr Behrndt, the change would ensure, among other things, that the EU, which was the first jurisdiction worldwide to develop and introduce a regulatory framework for biosimilars, “is not moving ahead in isolation” as it fine tunes its guidance in this area.
The EU is no longer alone in having a biosimilars framework, noted Mr Behrndt, who is deputy head of cabinet at DG Sanco, the commission’s Health and Consumer Policy Directorate General, which is responsible for pharmaceutical regulation. “Biosimilar production and research is now a global business,” he said.
The EGA has been lobbying for this change for some time. It knew things were moving in the direction it wanted, but this was the first time that a commission official had confirmed in public that it seemed that revising the guideline would suffice and that the fundamental legislation did not need changing.
Mr Richardson confirmed that the matter of a common reference product had “been on the horizon for a long time”. Health Canada was one of the first to move on the issue10 and so were “pioneers in that aspect”, he commented.
The EGA is hoping that other regulators follow the Canadian and now also the EU and US approach. The current requirement, Ms Kox told Scrip Regulatory Affairs immediately after the conference, is “unethical, uneconomical and an enormous burden that threatens the viability of this young industry”. The same principle applies in other developed and highly regulated markets and, the EGA comments in a position paper on the matter11, means that “biosimilar product applicants must repeat all clinical studies against reference biological products sourced from each territory in which they seek approval, even in circumstances where these reference biological products are identical, authorised to the same marketing authorisation holder or its affiliate, licensee or contractual partner and even manufactured in the same facility”.
“Such multiplication of studies is unnecessary… and in some ways represents a higher threshold for approval than that of the originator reference product applicant, who can seek approval in all territories based on the same data set from one global development programme.”
The EGA had been asking specifically for section 2.2 of the guideline, on Choice of Reference Product, to be revised in order to enable the EMA’s scientific committee, the CHMP, “to make a case-by-case, science-based assessment of the acceptability of the use of an identical reference biological product in appropriate circumstances”. Among other things, it wants the following text to be deleted:
Data generated from comparability studies with medicinal products authorised outside the Community may only provide supportive information.
and the following text to be added:
In circumstances where the reference medicinal product as described above has been authorised outside the Community to the same marketing authorisation holder or its affiliate, licensee or contractual partner, it may be possible for the applicant to also use the non-Community sourced reference medicinal product in the various comparability studies
Scientific bridging studies will always be required, Ms Kox explained12: the EU legislation requires reference to an EU-licensed reference product and so "bridging is essential to stay within the EU framework".
A rigorous physicochemical and biological comparison will always be performed with the EU licensed and EU sourced reference, the non-EU reference product and the biosimilar product, Ms Kox said. If the result is positive, comparative Phase III trials would be performed with the reference product from one region only.
Overarching guideline revision
The comment period on the concept paper outlining the commission’s proposals for change to the overarching guideline, CHMP/437/04, closed at the end of February this year. A revised draft guideline was originally expected to be issued for public consultation in the first half of this year but, said Mr Richardson, this is now due in the third quarter. There were comments from 11 stakeholders, and experts from the EMA/CHMP Biosimilar Medicinal Products Working Party and Biologics Working Party (BMWP) are currently working on the draft revised guideline.
Mr Richardson, who is section head biologicals in the human medicines development and evaluation unit (quality of medicines sector) at the EMA, gave a summary of stakeholder comments as follows:
support for clarification of terminology;
include a discussion on global development, follow same approach as the FDA (as discussed extensively above);
use EMA website rather than the guideline as a tool for updated list of reference documents;
safety and efficacy aspects would be better covered by the general and product specific clinical/non-clinical guidelines;
“generic approach” for biologicals is not appropriate and not necessary, sufficient flexibility provided in article 10(4); and
pharmaceutical form, strength and route of administration should normally be the same as for the reference product (although liquid versus lyophilised formulation should be acceptable).
Global reference product
Another presenter at the symposium, Cornelia Ulm, senior director regulatory affairs – biologics at Mylan and vice chair of the EBG, told the symposium that countries that wanted to provide affordable medicines to patients could not ignore the need for global development and that acceptability of a global reference product was critical to such development.
Global acceptability of data using a reference product sourced in one of three jurisdictions of the regions of the International Conference on Harmonisation was “a must”, Ms Ulm said.
In her presentation at the symposium on Japan's biosimilar guidelines, Teruyo Arato of the Office of Regulatory Science at the Pharmaceuticals and Medical Devices Agency noted that two biosimilars have been approved to date in Japan, the other ICH jurisdiction in addition to the EU and the US. Dr Arato, who stressed in her presentation that she was speaking in a personal capacity, made it clear that Japan was keen to be part of the global development discussion. The PMDA official seemed positive on the proposed EU/US approach, although it seems that there has been no official decision on the matter from the agency itself.
In addition to there being a need for global consensus on regulatory data requirements, there is a need also for “further rolling out” of the World Health Organization biosimilar guidelines13 that contain the same key concepts as already laid down in existing regional guidelines in countries such as the EU, Japan and Canada, Ms Ulm said. In this specific area, she highlighted WHO plans such as building technical expertise in national regulatory agencies worldwide in the area of biosimilar product assessment. [The WHO is running a three-day guidelines implementation workshop on in China at the end of May14. This will be followed by an informal two-day consultation on the revision of WHO recommendations to assure the quality, safety and efficacy of biological products prepared by recombinant DNA technology15.]
Other topics
Many other topics and questions were covered or raised on the second day of the symposium on April 20, either in presentations or during panel discussions. They included:
the numbers of biosimilars to have been through the approval system in the EU;
terminology;
the status of other EU biosimilar guidelines; and
the recently released draft US biosimilar guidances.
Numbers
Mr Richardson of the EMA provided details on review activities regarding marketing authorisation applications for biosimilars at the EMA overall. Of the 19 marketing authorisation applications that the agency has reviewed, 14 have been positive, four were withdrawn and one was negative. Thirteen biosimilar medicinal products currently hold a valid marketing authorisation (two somatropins, five epoetins and six filgrastims) and five biosimilar marketing authorisation applications are under review (three insulin human, one follitropin alfa and one infliximab). [Infliximab is the first monoclonal antibody to be reviewed – Ed.]
Terminology
Martina Weise of German drugs and medical devices regulator BfArM and vice chair of the EMA/CHMP BMWP (biosimilars working party) told the meeting she believed there was a need to promote, among other things, the use of consistent terminology in the area of biosimilars.
Misunderstanding of the biosimilar concept and inconsistent use of terminology have led to unsubstantiated fears about efficacy and safety of biosimilars in the EU, said Dr Weise. Not only are different terms in use around the world – biosimilars, follow-on biologicals, subsequent-entry biologicals, similar biopharmaceuticals, me-too biologicals, biogenerics or non-innovator proteins – there are different interpretations of the same term in different geographical regions, she noted.
Dr Weise called for: the proper communication of the biosimilar concept; globally agreed scientific principles; and the use of consistent terminology.
Other EU biosimilar guidelines/documents
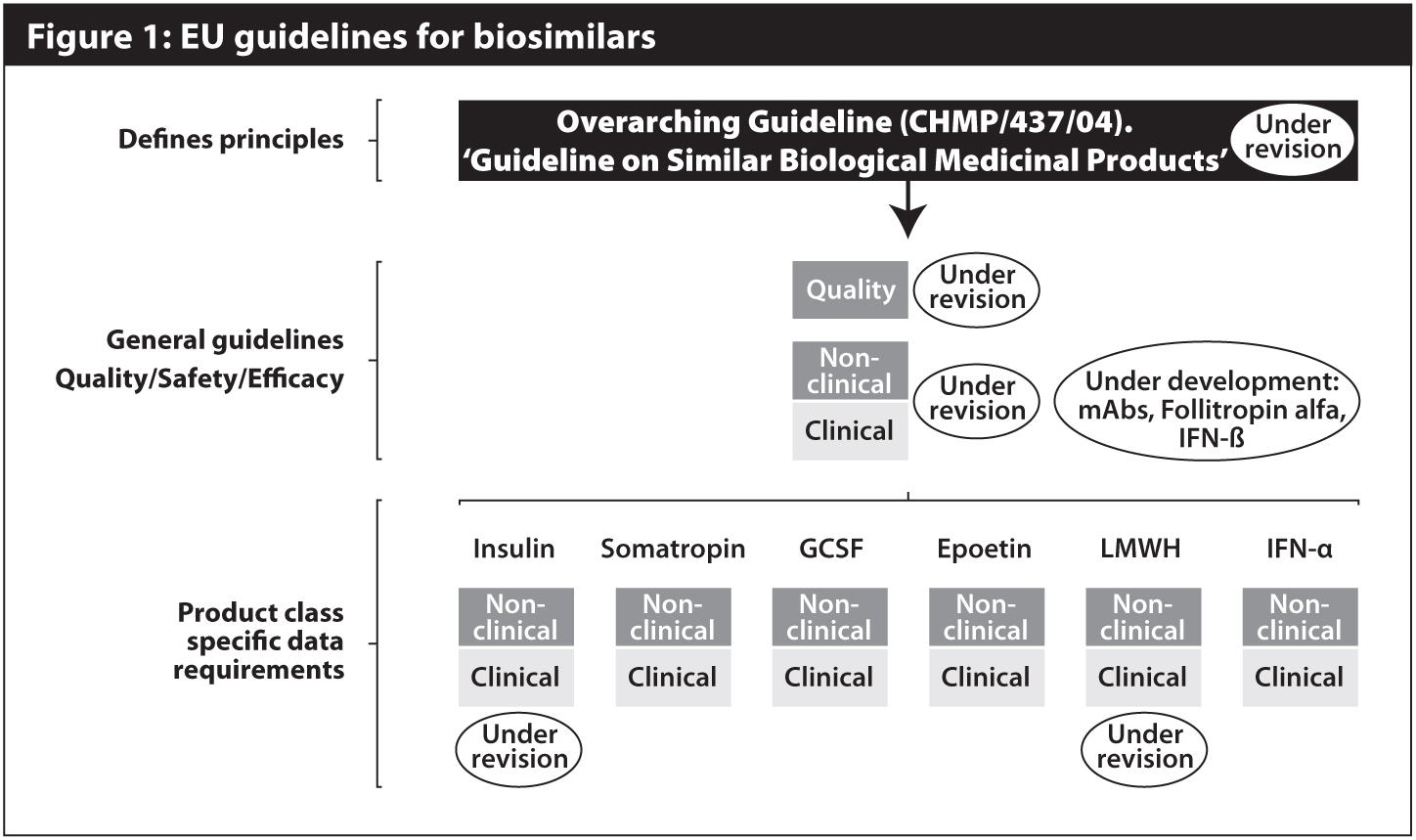
The EMA's Peter Richardson and Christian Schneider of the Danish Health and Medicines Authority and of the EMA/CHMP biosimilars working party gave an update on the status of other EU biosimilar guidelines, including those under revision and those under development (see Figure 1).
Monoclonal antibodies and immunogenicity assessment
Finalised EMA guidelines on biosimilars containing monoclonal antibodies (mAbs) and on immunogenicity assessment of mAbs are likely to be issued together in late May or in June16.
There was “huge interest” in the draft of the mAbs guideline17, Dr Schneider told the symposium. The EMA received 405 pages of comments from 22 stakeholders during the public consultation, which ended in May 2011. More than 180 pages of comments were received from 11 stakeholders on the draft guideline on immunogenicity assessement18, which was also out for comment until last May.
The EGA has described mAbs as "the next frontier" in the field of biosimilars.
Non-clinical/clinical & quality guidelines
Both general biosimilars guidelines – on non-clinical and clinical issues19,20 and on quality issues21,22 – are under revision, Mr Richardson noted.
The consultation on the concept paper on the revision of non-clinical and clinical issues guideline ended in December 2011. There were comments from 12 stakeholders and publication of a formal draft guideline is expected in Q3.
Four stakeholders submitted comments on the concept paper on the revision of the quality issues guideline, whose consultation period ended in May 2011. The CHMP adopted a formal draft guideline in April and the next step is for this document to be released for a six-month public consultation.
Product-specific guidelines
Of the six existing product-specific guidelines, on insulin, somatropin, GCSF, epoetin, low molecular weight heparin and interferon alpha, those covering insulin23,24 and LMWH25,26 are being revised. The consultation periods on concept papers relating to the revision of both guidelines ended in 30 September 2011.
In addition, product-specific guidelines are under development for biosimilars containing follitropin alfa27 and interferon beta28 (as well as for mAbs, as detailed above). The comment period on the relevant draft guidelines closes at the end of May.
Q & A document
The EMA’s question and answer document on biosimilars for the general public that was first published in 2008 is being updated. Key issues that are being considered for the update include: how similar are biosimilars; how safe are they and how are they evaluated; and can they be used interchangeably? Interchangeability as regards biosimilars was not within the remit of the EMA but the topic comes up frequently and “it is important to have recognition of that”, Mr Richardson said.
QRD guidance
General guidance on the quality review of documents (QRD)29 was also being updated, to include information relevant – but not specific – to biosimilars. It would, Mr Richardson said, provide general principles for the preparation of the summary of product characteristics (SmPCs) for generics, hybrids and biosimilars.
Due for publication “in the coming months”, the guidance will state that the QRD template and the general SmPC guideline30 should be applied to the generic/hybrid/biosimilar SmPC as far as possible.
Also, Mr Richardson said, format changes to the SmPC of the generic/hybrid/biosimilar in comparison to the SmPC of the reference medicinal product will be considered acceptable as long as the content remains the same for the relevant common information. This flexibility is aimed at addressing the fact that a diverse range of SmPCs can exist for the reference product where the reference product was approved on a national basis.
SmPCs and “biosimilar specific” information
Mr Richardson referred to the debate over what “biosimilar specific” information should be included in the biosimilar SmPC over and above what is cut and pasted from the SmPC for the original product.
There is a need for a consistent approach on this, Mr Richardson said, noting that the matter is under consideration by the EMA/CHMP biosimilars working party.
The current version of the SmPC guideline states only that Section 5.1 of the SmPC, on pharmacodynamic properties, should include a statement specifically saying that the product in question is a biosimilar. The working party will consider whether any additional information, for example regarding the comparability exercise that has been conducted in relation to the reference product, should also be included, Mr Richardson confirmed to SRA31. The innovator industry has been pushing for this32,33.
Procedural guidance for biosimilars
Lastly, Mr Richardson noted that the CHMP in February adopted procedural guidance covering specific aspects of biosimilars34; procedural guidance was already available for innovative products and for generics/hybrids. The new guidance – in the form of a Q&A – covers issues such as: eligibility; legal basis (Article 10 (4) of Directive 2001/83/EC); the international nonproprietary name; the reference medicinal product; and the comparability exercise.
The draft US biosimilar guidances
The presentation from John Pakulski, senior director and head of US biopharmaceutical regulatory affairs at Sandoz Inc and chair of the US Generic Pharmaceutical Association’s biosimilars task force, covered the three draft US FDA guidance documents on biosimilars that were issued in February35.
Implemented appropriately, Mr Pakulski said, the guidelines could lead to “a successful regulatory pathway” for biosimilars. However, there were several important questions:
Will comprehensive and robust analytical data yield meaningful reductions in clinical and immunogenicity studies?
What amount of bridging data will be required to support use of a foreign-sourced reference product comparator?
How flexible will the FDA be regarding clinical trial designs?
Will immunogenicity study requirements focus on clinically meaningful immunogenicity endpoints? and
To what extent will extrapolation of indications be supported by the FDA without additional clinical trials or further elucidation of mechanism?
In addition, the GPhA hopes that the “sequential” – ie step-by-step – development approach that the FDA is recommending and the fact that the agency is advising companies to “consult extensively” throughout the process with the FDA will not affect companies’ ability “to timely develop biosimilars”. Mr Pakulski said he hoped that it would be possible, for example, to conduct the various steps in parallel.
Other topics or areas discussed at the symposium included the following:
the need for Japan to be more directly involved in international discussions on biosimilars regulation (the EU and the US already work closely together in this area);
regulatory approaches to follow-on biologics in major Latin American markets (namely Mexico, Brazil, Argentina and Peru);
the possibility of biosimilars having indications additional to those of the originator product;
the paucity of intellectual property incentives to develop new indications; and
interchangeability and substitution.
References
1. Guideline on similar biological medicinal products (CHMP/437/04), 30 October 2005, www.emea.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003517.pdf
2. EMA, Concept paper on the revision of the guideline on similar biological medicinal product (EMA/CHMP/BMWP/572643/2011), 24 November 2011, www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2011/11/WC500117987.pdf
3. EMA proposes revision of its overarching biosimilars guideline, Scrip Regulatory Affairs, 1 December 2011
4. Biosimilar Medicines: 10th EGA International Symposium, 19-20 April 2012, London (UK), www.gpaconferences.com/files/BIOS12_Programme.pdf
5. Scientific Considerations in Demonstrating Biosimilarity to a Reference Product, 9 February 2012, www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM291128.pdf
6. Quality Considerations in Demonstrating Biosimilarity to a Reference Protein Product, 9 February 2012, www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM291134.pdf
7. Biosimilars: Questions and Answers Regarding Implementation of the Biologics Price Competition and Innovation Act of 2009, 9 February 2012, www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM273001.pdf
8. US FDA issues much-awaited draft guidelines on developing biosimilars, Scrip Regulatory Affairs, 10 February 2012
9. Personal communication, Suzette Kox, 2 May 2012
10. Health Canada finalises guidance on biosimilars, Scrip Regulatory Affairs, 18 March 2010
11. EGA position on choice of reference medicinal product in biosimilar development, June 2011
12. See Reference 5
13. WHO guidelines on evaluation of similar biotherapeutic products (SBPs), Expert committee on biological standardization, 19-23 October 2009, www.who.int/biologicals/areas/biological_therapeutics/BIOTHERAPEUTICS_FOR_WEB_22APRIL2010.pdf
14. WHO Workshop on implementing WHO Guidelines on evaluating similar biotherapeutic products (SBPs), Xiamen, China, 28-30 May 2012, www.who.int/biologicals/areas/en/
15. WHO Informal Consultation on the revision of the recommendations to assure the Quality, Safety and Efficacy of biological products prepared by recombinant DNA technology, Xiamen, China, 31 May-1 June 2012, www.who.int/biologicals/areas/en/
16. Personal communication, Christian Schneider, Danish Health and Medicines Authority & EMA/CHMP BMWP, 1 May 2012
17. Draft guideline on similar biological medicinal products containing monoclonal antibodies, EMA/CHMP/BMWP/403543/2010, 18 November 2010, www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2010/11/WC500099361.pdf
18. Draft guideline on immunogenicity assessment of monoclonal antibodies intended for in vivo clinical use, 18 November 2010, EMA/CHMP/BMWP/86289/2010, www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2010/11/WC500099362.pdf
19. Concept paper on the revision of the guideline on similar biological medicinal products containing biotechnology derived proteins as active substance: non-clinical and clinical issues, EMA/CHMP/BMWP/572828/2011, 22 September 2011
www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2011/10/WC500115611.pdf
20. Guideline on similar biological medicinal products containing biotechnology-derived proteins as active substance: non-clinical and clinical issues, EMEA/CHMP/BMWP/42832/2005, 22 February 2006, www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003920.pdf
21. Concept paper on the revision of the guideline on similar biological medicinal products containing biotechnology-derived proteins as active substance: quality issues, EMA/CHMP/BWP/617111/2010, 7 February 2011, www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2011/02/WC500102285.pdf
22. Guideline on similar biological medicinal products containing biotechnology-derived proteins as active substance: quality issues, EMEA/CHMP/BWP/49348/2005, 22 February 2006, www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003953.pdf
23. Concept paper on the revision of the guideline on nonclinical and clinical development of similar biological medicinal products containing recombinant human insulin,
EMA/CHMP/BMWP/506470/2011, 21 July 2011, www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2011/07/WC500109587.pdf
24. Annex to guideline on similar biological medicinal products containing biotechnology-derived proteins as active substance: non-clinical and clinical issues - Guidance on similar medicinal products containing recombinant human insulin, EMEA/CHMP/BMWP/32775/2005, 22 February 2006,,www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003957.pdf
25. Concept paper on the revision of the guideline on nonclinical and clinical development of similar biological medicinal products containing low-molecular-weight heparins, EMA/CHMP/BMWP/522386/2011, 21 July 2011,
www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2011/07/WC500109588.pdf
26. Guideline on similar biological medicinal products containing low-molecular-weight-heparins, EMEA/CHMP/BMWP/118264/2007, 19 March 2009, www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003927.pdf
27. Draft guidelines on similar biological medicinal products containing recombinant follicle stimulation hormone, CHMP/BMWP/671292/2010, www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2011/11/WC500117986.pdf
28. Draft guidelines on similar biological medicinal products containing interferon beta, EMA/CHMP/BMWP/652000/2010, 15 December 2011, www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2012/01/WC500120652.pdf
29. Quality Review of Documents, EMA website, accessed 11 May 2012, www.ema.europa.eu/ema/index.jsp?curl=pages/regulation/document_listing/document_listing_000254.jsp&mid=WC0b01ac058008c34c
30. European Commission guideline on summary of product characteristics (SmPC) Revision 2, September 2009 (posted 21 October 2009), http://ec.europa.eu/health/files/eudralex/vol-2/c/smpc_guideline_rev2_en.pdf
31. Personal communication, EMA, 10 May 2012
32. Overview of comments received on draft Revision 2 of the SmPC guideline, EMEA/CHMP/663087/2009, 22 October 1009, www.ema.europa.eu/docs/en_GB/document_library/Regulatory_and_procedural_guideline/2009/12/WC500027105.pdf
33. EBE-EFPIA Position paper on labelling of biosimilar medicinal products, 28 March 2007 www.biofarmacos.org/files/contenido/Ebe-Efpia_Biosimilars_labelling_position_paper_28_mar_07.pdf
34. Q&A: Similar biological product applications, www.ema.europa.eu/docs/en_GB/document_library/Regulatory_and_procedural_guideline/2012/04/WC500125166.pdf (see also EMA Procedural advice for users of the centralised procedure for similar biological medicinal products applications, EMA/940451/2011, November 2011, www.emea.europa.eu/ema/index.jsp?curl=pages/regulation/general/general_content_000529.jsp&mid=WC0b01ac0580533e0b)
35. See References 5-8 and also Drug makers have their say on US FDA's proposed biosimilars guidances, Scrip Regulatory Affairs, 18 April 2012
Maureen Kenny is the editor of Scrip Regulatory Affairs.