Court Order Could Convolute Emergency Contraception Market
This article was originally published in The Tan Sheet
Executive Summary
A federal appeals court mandates full OTC sales of two-tablet levonorgestrel products, as requested in a 2001 citizen petition. The order delineates two-dose emergency contraceptives from Teva’s Plan B One-Step product, which did not exist at the time of the petition.
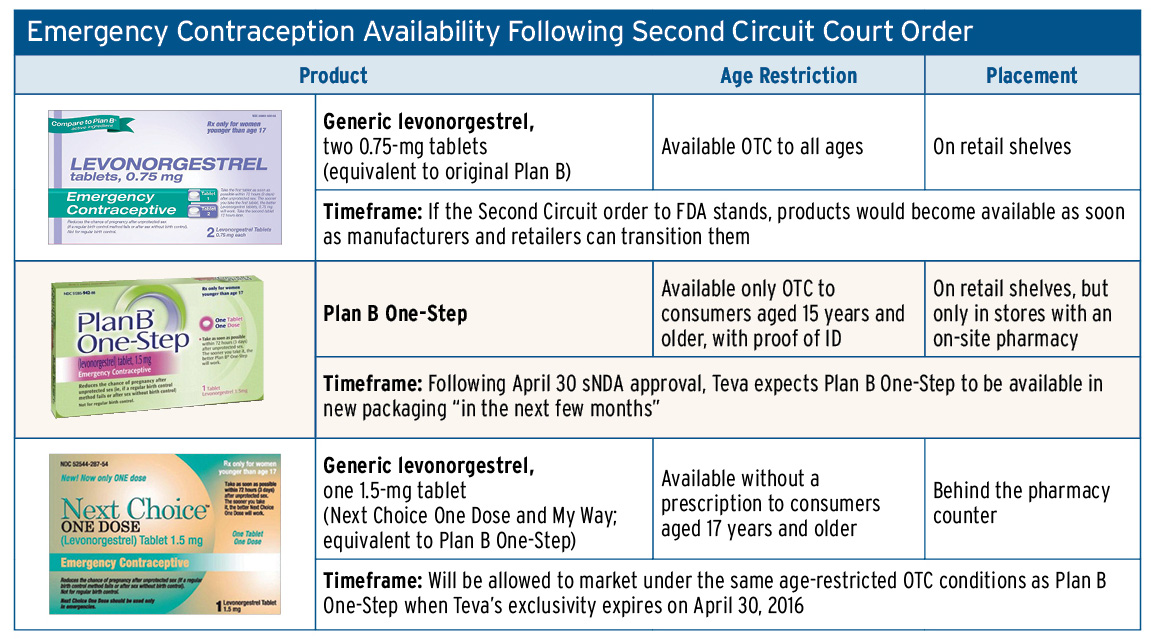
A complex, multi-tiered system for marketing emergency contraception could emerge following a June 5 appellate court order that mandates unrestricted OTC sales of generic versions of the original two-dose Plan B product.
In ongoing litigation initiated by reproductive health advocacy groups, the U.S. Court of Appeals for the Second Circuit denied the Department of Justice’s motion for a stay of the district court order directing FDA to approve a citizen petition that sought full OTC availability of all levonorgestrel-based emergency contraceptives (Also see "In Brief" - Pink Sheet, 3 Jun, 2013.).
However, the appellate court delineated the generic two-dose (0.75mg) products from Teva Women's Health Inc.’s Plan B One-Step, opting to grant the government’s request for a stay of the lower court’s order, pending appeal, as relates only to Plan B One-Step. Teva’s single-dose (1.5mg) product did not exist when the citizen petition originally was submitted to FDA in 2001.
According to attorneys for plaintiff the Center for Reproductive Rights, DoJ has until June 19 to appeal the denied portion of its stay motion to the Second Circuit’s full panel. Should that fail, the final option would be an appeal to the Supreme Court – specifically, to Justice Ruth Bader Ginsburg, who has responsibility for Second Circuit appeals.
Barring successful government appeals, however, consumers seeking emergency contraception could face a confusing scenario: generic two-dose levonorgestrel products will be sold at retail to consumers of all ages, while Plan B One-Step also will be shelved as an OTC but restricted to consumers aged 15 years and older under FDA’s recent approval of Teva’s supplemental new drug application.
In May, district court Judge Edward Korman blamed FDA for “putting in place a convoluted triple-tiered marketing scheme that will only increase the confusion that already prevents women from obtaining timely access to emergency contraceptives.”
Generic products likely also will have the benefit of being sold at a lower price point than the branded competition.
Perrigo Co. PLC, Lupin Ltd. and Allergan PLC each has an approved abbreviated new drug application for nonprescription two-dose levonorgestrel products, though Actavis has transitioned its branded generic product to Next Choice One Dose following the 2012 approval of its ANDA for the single-tablet version.
Now, Next Choice One Dose and Gavis Pharmaceuticals LLC’s My Way Emergency Contraceptive, the only approved equivalents to Plan B One-Step, could become the most difficult of all emergency contraceptives to purchase. These branded generics potentially will remain relegated to pharmacy-only sales and available nonprescription to consumers aged 17 and older until Teva loses market exclusivity in 2016; consumers younger than 17 still require a prescription for these products (Also see "Plan B One-Step Goes OTC, With Three-Year Exclusivity, Lower Age Limit" - Pink Sheet, 6 May, 2013.).
After Judge Edward Korman ruled all levonorgestrel-based contraceptives must move over the counter, DoJ attorneys argued in U.S. District Court for the Eastern District of New York that a stay of Korman’s order was necessary to prevent potential market confusion in case a successful appeal wound up returning products behind the counter (Also see "FDA Moves To Appeals Court In Plan B Case" - Pink Sheet, 13 May, 2013.).
Korman denied the stay motion on May 10, noting that FDA largely was to blame for “putting in place a convoluted triple-tiered marketing scheme that will only increase the confusion that already prevents women from obtaining timely access to emergency contraceptives.”
For now, reproductive health advocates, who had sued FDA over OTC access to emergency contraception, celebrated another apparent milestone.
“Finally, after more than a decade of politically motivated delays, women will no longer have to endure intrusive, onerous and medically unnecessary restrictions to get emergency contraception,” said Nancy Northup, CEO of the Center for Reproductive Rights.
A DoJ spokeswoman declined to comment, saying only that the department is reviewing the court’s order.