McNeil Recalls Liquid Infants’ Tylenol With Dosing Device Problem
This article was originally published in The Tan Sheet
Executive Summary
McNeil Consumer Healthcare recalls an Infants’ Tylenol product that returned to market in November, after receiving consumer complaints about a problem with the SimpleMeasure dosing device system. The recall marks a misstep in the J&J subsidiary’s comeback from OTC quality control issues.
An apparent defect related to the dosing devicepackaged withanInfants’ Tylenol product promptedMcNeil Consumer Healthcare’s latest liquid pediatric OTC drug recall.
The recall suggests the difficulty pharma firms face in improving OTC dosing accuracy and safety while providing user-friendly dosing systems.
The Feb. 17 retail- and wholesale-level recall of an overhauled product launched in November also marks a misstep in McNeil’s comeback from quality control issues that led to extensive OTC recalls in 2009 and 2010. However, the Johnson & Johnson subsidiary can point to this latest precautionary recall as a sign it has become more responsive to consumer complaints.
McNeil recalled 574,000 bottles of the 1-ounce oral suspension acetaminophen product in grape flavor. At issue was the flow restrictor at the top of the bottle’s neck – part of the firm’s new SimpleMeasure dosing system – which some consumers accidentally pushed into the bottle when inserting the tip of the accompanying plastic syringe.
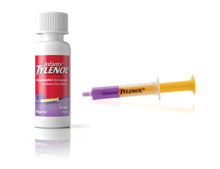
Infants' Tylenol with SimpleMeasure dosing system
Image courtesy of Johnson & Johnson
The company said it received 17 complaints about the dosing system, but consumers can continue using the Tylenol product as long as the flow restrictor remains in place. McNeil is offering a refund to unsatisfied consumers who purchased the pain reliever/fever reducer, which has a suggested retail price of $6.99.
McNeil President Denice Torres said in a same-day letter to J&J employees that the recall is a “disappointment” to McNeil, which she characterized as still recovering from the liquid OTC recalls that affected the Tylenol, Benadryl, Motrin and Zyrtec brands (Also see "Andrx Concerta" - Pink Sheet, 29 Mar, 2004.).
The firm has begun returning to market many of the products it pulled from shelves nearly two years ago, with more expected throughout 2012.
Torres told her J&J colleagues McNeil is “making steady progress” while working through the consent decree with FDA under which one of its manufacturing facilities is closed and two others are operating during remediation processes.
“All of this work will result in the re-emergence of a world-class OTC company,” she said.
Flow Restrictors Prove Fallible
J&J registered a trademark for the SimpleMeasure name in April 2011 and launched the dosing system with the Infants’ Tylenol later that year.
The dosing system represents J&J’s own approach to improving OTC dosing accuracy and safety, which has developed in recent years as a priority across FDA, industry, consumer groups and the academic community (Also see "FDA Gets Strict On Liquid OTC Dosing Devices In Draft Guidance" - Pink Sheet, 9 Nov, 2009.).
In a message to consumers about the recall, McNeil said it created SimpleMeasure to ease measuring an appropriate dose. The flow restrictor and syringe were manufactured at a J&J plant in Latina, Italy, a company spokeswoman said in an email.
In conjunction with the OTC industry’s recent voluntary move to a single concentration of pediatric acetaminophen products, the Consumer Healthcare Products Association announced in May all liquid acetaminophen for infants and children would come with flow restrictors to minimize access to the drug in case of accidental unsupervised ingestion (Also see "Acetaminophen Panel To Mull Expanded Dosing, Standard Solid Concentration" - Pink Sheet, 16 May, 2011.).
But the Institute for Safe Medication Practices points out even flow restrictors are not foolproof. The patient safety organization, in its Dec. 15 Medication Safety Alert, noted parents still must remember to store drug products with the safety cap in place, or a child still could ingest the medication by prying off or unscrewing certain flow restrictors.
Screw-on flow restrictors “may attract young children because they often look like the top of a sippy cup or infant bottle,” ISMP said.
ISMP said it found that most infant OTC acetaminophen products on the market come with restrictors that plug into the neck of the bottle and are difficult to pry out.
Centers for Disease Control and Prevention official Daniel Budnitz hopes the SimpleMeasure issue does not throw a wrench in the movement toward more “exposure-limiting” designs for pediatric drug products. Budnitz is director of CDCs’ medication safety program and leads the Preventing Overdose and Treatment Errors in Children Task Force, which includes CDC, drug companies, academics and other stakeholders.
“I think this recall underscores the challenges involved in improving packaging and hope that this does not discourage manufacturers from developing new safety-conscious and innovative packaging, but rather encourages them to continue their packaging improvement efforts,” he said in an email.
Budnitz pointed out that some liquid OTC dosing device designs incorporate the flow restrictor as a molded part of the bottle. But regardless of the design, he encouraged firms to conduct testing on how children and adults will actually use the packaging components.