Comparative Effectiveness Research and the Environment for Health Care Decision-Making
This article was originally published in RPM Report
Executive Summary
During the last five years, there has beenenormous changes in the health care environment: the implementation of the Affordable Care Act and its many provisions, including the creation of PCORI; the development of health exchanges and a move toward value-based payments, most notably in Medicare programs. In the midst of all this change, what impact will comparative effectiveness research have on health care decision-making?
For the last five years the National Pharmaceutical Council has conducted an annual survey that provides a snapshot of health care stakeholders’ perceptions of the key players involved in the comparative effectiveness research (CER) process, from setting priorities to translating and disseminating the research findings.
A few survey findings have become clearer with each year: CER is important, the Patient Centered Outcomes Research Institute (PCORI) is the dominant player in this space, and the goalposts for when we will see significant impact from CER are—still—three to five years down the road.
Dan Leonard is the president of the National Pharmaceutical Council.
Why does CER matter? First, there has been an enormous public and private investment in CER, which is meant to provide better information about different treatment alternatives. Second, in the hands of patients, providers, payers and other stakeholders, this data should help to improve decision-making about treatments, coverage decisions, delivery system models and other issues affecting health care quality and outcomes.
Exhibit 1
Source: National Pharmaceutical Council
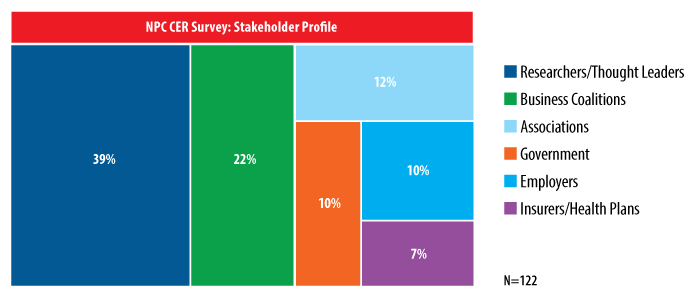
To check the pulse of stakeholders, or those who are working in the CER field or are familiar with the concept, NPC surveyed representatives from the following categories: researchers/thought leaders, government, associations, business coalitions, employers and insurers/health plans. We received 122 responses.
Exhibit 2
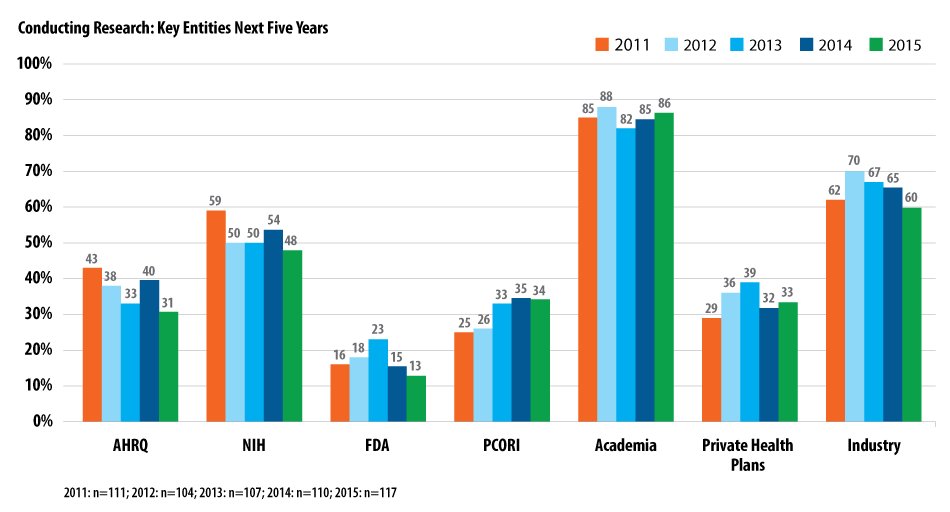
Source: National Pharmaceutical Council
Taking a closer look, more than 90 percent of the survey respondents said that CER is “very” or “somewhat important.” Interestingly, this view has been consistent across the years. (Also see "Impact of CER Still on the Horizon, Say Health Care Stakeholders" - Pink Sheet, 23 May, 2014.)
Although these respondents view CER as important, its impact has not yet been felt. “Moderate” or “substantial improvement” in decision-making because of CER won’t likely be felt for another three (83%) to five years (93%).
It’s clear that PCORI is now considered the dominant player in the CER space. During the last few years, PCORI has greatly raised its public profile by awarding more than $700 million in research grants, creating advisory councils and maintaining an ongoing dialogue with patients and advocacy organizations.
As a result, PCORI (75%), the National Institutes of Health (NIH) (63%) and the Agency for Healthcare Research and Quality (AHRQ) (62%), respectively, are viewed as leaders in setting the research priorities that will be addressed by CER.
Exhibit 3
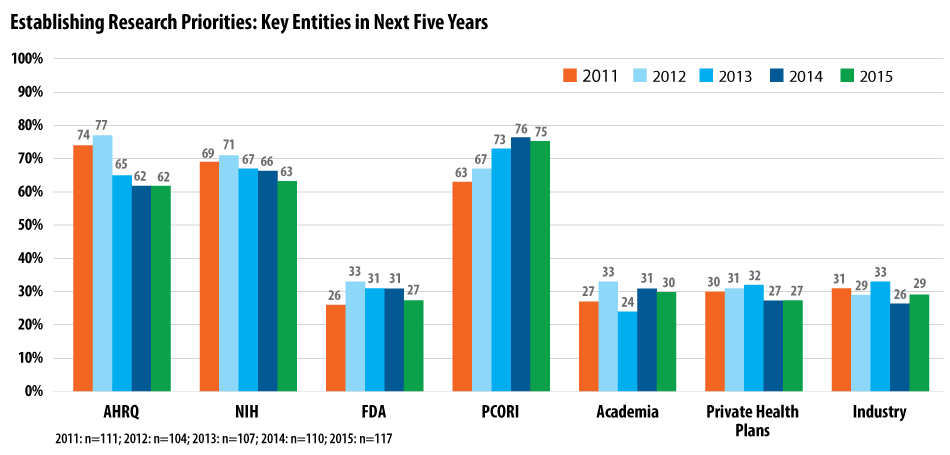
Source: National Pharmaceutical Council
The same three organizations, in a slightly different order, are seen as playing keys roles in establishing research standards. Here, PCORI is still on top at 77%, possibly because it requires its grant recipients to follow the research standards set out in its methodology report. AHRQ (68%) was next, followed by NIH and academia, both at 50%.
Exhibit 4
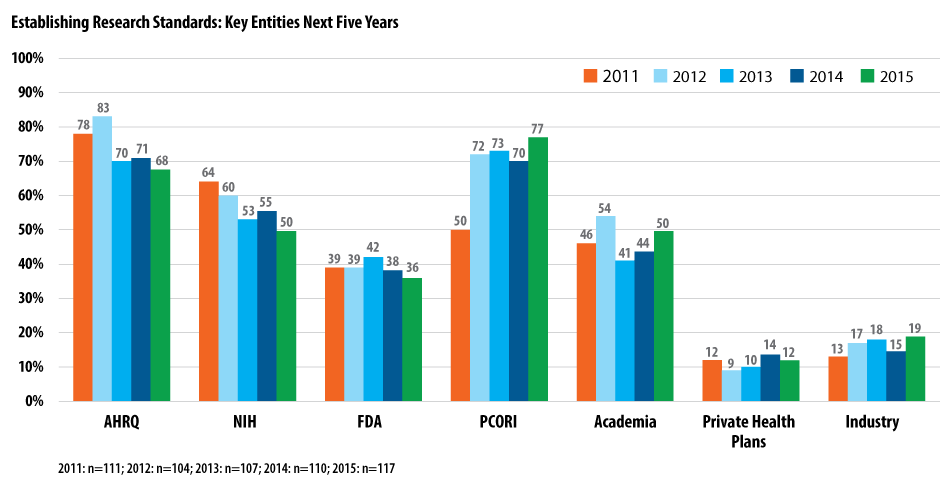
Source: National Pharmaceutical Council
PCORI also was recognized by respondents as the key organization in funding and monitoring research (81%), followed by NIH (73%) and the biopharmaceutical industry (65%), which makes large investments in research.
Exhibit 5
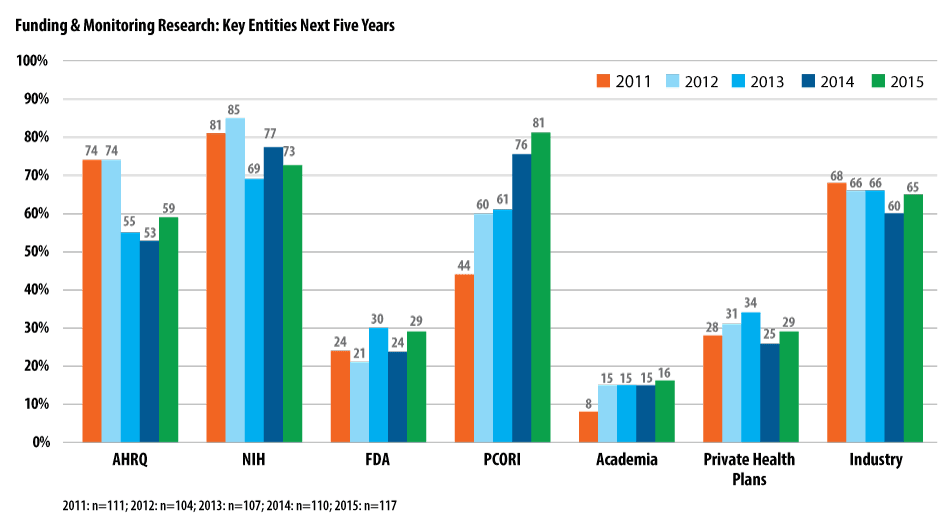
Source: National Pharmaceutical Council
Which groups are most likely to conduct the studies? According to respondents, it’s academia (86%), which has been awarded a large percentage of grants, followed by the biopharmaceutical industry (60%) and NIH (48%).
Following the completion of these studies, the research needs to be translated and disseminated to health care decision-makers. AHRQ, tasked by federal law to disseminate PCORI’s work, is viewed as the lead entity (78%), followed by PCORI (69%) and academia (60%).
Exhibit 6
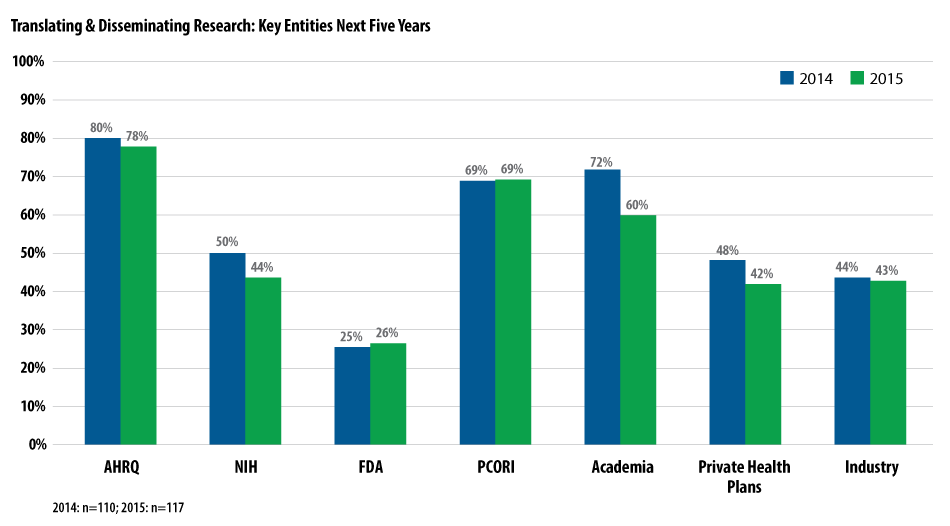
Source: National Pharmaceutical Council
Consistent Results as ACA Implementation Takes Hold
All in all, these views have been fairly consistent during the last few years, perhaps indicating that the roles are becoming more defined now that the Affordable Care Act has been implemented.
We also asked respondents a few questions about the health care decision-making environment, considering factors that could impact the quality of comparative effectiveness research and its usefulness in making treatment and coverage decisions. Here’s what our respondents said:
- There is a growing trend toward agreed upon research methods, which would provide more consistency in the conduct and evaluation of CER.
- Despite the growing focus on patient-centered care, only 41% of stakeholders felt that research priorities adequately addressed the treatment choices faced by patients and providers.
- Less than half of respondents said that there is no or little transparency in the processes used by decision-makers to interpret evidence.
- Fifty-eight percent of respondents said that the value of treatments remains narrowly focused on only clinical effectiveness, rather than taking into account factors that matter to patients, such as quality of life, workplace productivity, adherence to treatments and other outcomes.
- Sixty-seven percent said that the evidence base is not complete enough to inform the choices faced by patients and providers.
- Forty-four percent felt that the use of real-world evidence in making health care decisions is still limited.
- When it comes to accounting for variability in patient responses, 43 percent of respondents felt that individual treatment effects are not taken into consideration.
While we’re seeing some changes in the landscape, it’s a slow evolution. Many of the CER projects that have been funded by the 2009 government stimulus, PCORI, NIH and other entities in recent years will be coming to fruition within the next one to two years. As those findings are disseminated, we’ll want to understand whether the findings on recommendations from that research will be taken up by stakeholders and if and when it will have an impact on health care decision-making.
And, as CER results become more prevalent, we’ll also want to monitor how the health care environment continues to change. Will our evidence base become more complete with this new information? How will real-world evidence be considered in decision-making? Will research priorities more adequately address patient needs? The environment is evolving, but as with CER, those changes are likely still down the road.