Europe’s Drug Shortages Demand Clear EMA Role & Political Will, DIA Told
This article was originally published in The Pink Sheet Daily
Executive Summary
Medicine shortages are a growing problem in Europe that need urgent political attention so that the region’s top regulator can gain the authority and resources to prevent new deficits, such as a searchable public database that lists drugs in shortage, attendees at the DIA’s 27th annual EuroMeeting were told in Paris.
Europe’s fractured regulatory landscape and lack of a central database of supply disruptions threaten to let drug shortages spread even further in the region, delaying or denying tens of thousands of patients the care they need, according to regulators, health care professionals and patient organizations.
“Medicine shortages are affecting patients all across the EU and beyond,” said François Houÿez, director of treatment information and access for the European Organization for Rare Diseases (EURORDIS). “Medicines of major therapeutic interest – and with no alternatives – are in short supply. Yet, the public health impact of shortages is not measured,” he told DIA’s 27th annual EuroMeeting.
Medicine shortages were a key theme at the conference, underscoring the growing threat to the region. The conference, held April 13-15 in Paris, brought together professionals from more than 50 countries in the biopharmaceutical industry, contract research and service organizations, academic research centers, regulatory agencies, health ministries and patient organizations.
Drug Deficits Mainly Dealt With At National Level In Europe
In the 28-nation European Union, most medicine shortages are dealt with at the local level by the national competent authorities. The region’s top drug regulator, the European Medicines Agency (EMA), can be involved only in certain situations, such as when a medicine shortage is linked to a safety concern or affects several member EU states. But there is no centralized database collecting information about drug shortages in European countries. And drug manufacturers do not have to warn European regulatory authorities of a likely shortage.
Also, there is no coordinated European action to mitigate shortages or to ensure that the remaining stock is targeted at the patients most in need. This is helping drug shortages to spread in the region, according to presentations made at the conference.
“The detrimental impact to patients of medicine shortages is very real,” said Roberto Frontini, president of the European Association of Hospital Pharmacists (EAHP). “Doses are missed, treatments are switched to less efficacious alternatives, and medical errors are caused as a result. Add to that the extra stress in a safety critical environment, the loss of man hours and the gross distraction shortages cause to the provision of other pharmacy services and you have a pan-European public health threat requiring action at the EU level.”
Europe “Needs Its Own FDASIA”
Frontini and other speakers said new powers and resources should be given the EMA to help it prevent and mitigate the impact of drug shortages similar to the mandate given the U.S. FDA in 2012 by the Food and Drug Administration Safety and Innovation Act (FDASIA).
Under FDASIA, drug manufacturers must report upcoming shortages to the FDA six months in advance, or as soon as they practically can, allowing FDA to take early preventative steps. FDA cannot use FDASIA to force manufacturers to produce an additional amount of a particular drug, but the law does give the agency access to information in advance, allowing it to alert health care providers before deficits happen. FDA has a searchable public database listing drugs in shortage, along with a daily information feed that includes updates from manufacturers. FDA says FDASIA has helped the agency to reduce drug shortages (Also see "Thanks to FDASIA: Drug Shortages Declining, FDA Says" - Pink Sheet, 11 Feb, 2015.).
“That’s what EMA should also have,” Frontini told a DIA panel discussion on the subject.
He said EAHP’s most recent survey of medicines shortages in European hospitals showed the problem is extremely widespread. Its latest poll, based on 600 responses from 36 European countries, showed 86% of respondents saying such deficits create difficulties in delivering care to patients and/or operating the hospital pharmacy. The poll – the biggest of its kind ever – showed 66% of respondents saying medicine shortages affect their hospital pharmacy on a daily or weekly basis.
Areas in which drug shortages are most commonly reported according to the survey are antimicrobial agents, oncology medicines, emergency medicines, cardiovascular medicines and pain-relief agents (see graphic).
European Drug Shortages Survey
Europe's Medicines Shortages: An Ongoing Problem
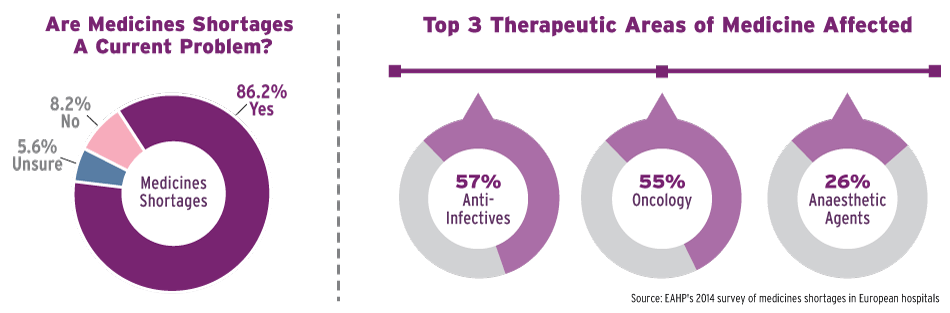
EAHP's 2014 survey of medicines shortages in European hospitals
Drug Shortages Occur For Many Reasons
Drug shortages happen for many reasons, often relating to manufacturing breakdowns at various stages of the supply chain, such as production problems, availability of raw materials, contamination, or a lack of components such as vials or containers. It affects all stakeholders of the health care system: patients, pharmacists, clinicians, the pharmaceutical industry and policy makers. A drug maker also can decide to stop producing a certain drug, perhaps for commercial reasons, such as switching production to a more profitable product. The supply side also can be influenced by policy measures, such as restricted drug production or allocation and quality requirements.
Causes of drug shortages in the EU might also stem from an unexpected rise in demand, unforeseen shifts in clinical practice, or from vendors seeking to profit from parallel trade. Through parallel trade, drug vendors can profit by shipping their stock from countries where prices are lower, such as Greece and Spain, to countries with higher prices, such as Germany. Other factors include globalization of manufacturing and distribution activities, notably to emerging markets, leading to fewer supply sites and less production flexibility.
EMA’s Steps Seen Lacking So Far
Following the publication of a reflection paper in November 2012 on shortages caused by manufacturing and quality issues, EMA together with EU member states developed a plan with defined actions to co-ordinate the assessment of shortages, develop risk-minimization measures to try to alleviate the impact on patients and facilitate communication on the issue within the EU regulatory network.
The EMA also has established an online catalogue of shortages that have been assessed by the EMA's Committee for Medicinal Products for Human Use (CHMP) and/or the Pharmacovigilance Risk Assessment Committee (PRAC). It is designed to communicate clear information to patients, health care professionals and other stakeholders on shortages assessed by the EMA.
Frontini said the EMA’s public shortage catalogue is a start but only offers limited value because it doesn’t offer a complete, up-to-date assessment of drug shortages in Europe, due to the limited criteria for inclusion.
He believes a comprehensive database needs to be established and run by the EMA. A framework should be put in place ensuring clarification and enforcement of legal responsibilities for suppliers to report likely disruptions and problems at early stages. Also, “enforceable criteria need to be put in place for a fair distribution of medicines in case of shortages, that’s based on patients’ needs and not on commercial interests,” Frontini said.
‘Responsibility Gap’ Needs To Be Filled
These needed reforms will only happen if responsibility to tackle this cross border health threat is taken on by the EU’s executive body, the European Commission. “A ‘responsibility gap’ at the EU level must also be overcome,” Frontini said.
Stakeholders hope EU health ministers will discuss the issue of drug shortages at their June meeting in Riga, Latvia. “We have made a request that this be put on their agenda and hope their meeting will give us a better understanding of the political will in Europe on this and eventually lead to a mandate for the Commission to carry out an enquiry and recommend what action should be taken,” said EURORDIS Director Houÿez.
EURORDIS and 44 other patient and health care groups are lobbying for the EMA to be given a clearer legal mandate. They also want a reporting mechanism established allowing health care professionals and patients to report product shortages. They say that as soon as a shortage seems possible, particularly for a product where this could have severe consequences for public health, manufacturers should have to inform the EMA and relevant health care professional and patient organizations, even if this may create false alerts in some cases.
The group says any company applying for marketing authorization for essential medicines should need to produce a supply-shortage risk-assessment plan as part of its application detailing how its production capacity will be able to satisfy demand, as well as explain what measures the company could take if demand is higher than anticipated, or if there are manufacturing problems. Another suggestion from the lobby group is that where shortages occur, the allocation of remaining supply to European countries should be decided by the EMA and its scientific committees, with consultation from health care professionals and patients.
Pharma Industry Role In Deficits A Focus
These points were also heard at the DIA Paris conference, with some delegates calling for more commitment from the pharma industry.
“Shortage-prevention plans by companies need to become part of their responsibility for the product,” said Lidia Retkowska-Mika, legal director at Poland’s Office for Registration of Medicinal Products, Medical Devices and Biocides. She said the drug sector needs to acknowledge that medicines are, by their very nature, unique commercial products. “Is the drug industry ready to accept that medicines are different - that they are not ‘just goods’ that are sold like any other commercial product?” she asked.
There is dialogue on the subject going on between European associations representing pharmacists, pharmaceutical wholesalers, the research-based pharmaceutical companies, the generics industry, the self-medication industry and parallel distributors. These talks have been going on for more than a year.
“The spirit of these discussions has been to avoid assigning blame to any particular stakeholder group, but instead to explore areas of common ground where European cooperation may bring benefits, in terms of easing the problem, or improving communication between stakeholders,” said Pär Tellner, a director at the Brussels-based European Federation of Pharmaceutical Industries and Associations.
“The stakeholders do not yet feel that discussions are sufficiently advanced to make a joint public statement on the issue. Discussions are progressing well, and in good spirit, and we expect to achieve a first set of results by the end of 2015,” he said.
