Angiomax vs. Heparin Booth Display Can’t Withstand Weight Of FDA Scrutiny
This article was originally published in The Pink Sheet Daily
Executive Summary
Although The Medicines Co. cited multiple studies to buttress superiority claims for its angina treatment Angiomax (bivalirudin), FDA found that none of these studies actually supported the idea that the drug is superior to heparin.
Assertions by The Medicines Co. that its anticoagulant Angiomax (bivalirudinis) is superior to heparin in the treatment of unstable angina fall short, despite what appears at first glance to be solid evidence from multiple studies, according to a “notice of violation” letter from FDA’s Office of Prescription Drug Promotion.
The April 13 letter, which OPDP posted to its web page April 27, critiqued TMC for a panel intended for a promotional booth at an unspecified professional conference. The agency said TMC had twisted the results of two non-inferiority studies to try to prove superiority – the “ACUITY PCI” and “REPLACE-2” clinical trials.
ACUITY-PCI enrolled patients with unstable angina and non-ST segment myocardial infarction undergoing percutaneous coronary intervention to receive enoxaparin or unfractionated heparin (UFH) plus glycoprotein IIb/IIIa inhibitor (GPI), Angiomax plus GPI or Angiomax alone. This study actually “failed to show non-inferiority of Angiomax to enoxaparin or UFH when analyzed for the endpoint of death or MI at 30 days,” FDA noted. REPLACE-2, which supported Angiomax’s approval for patients undergoing PCI, included patients undergoing PCI with unstable angina, myocardial infarction within seven days prior to intervention, stable angina, and positive ischemic stress test.
“Non-inferiority trials are not designed to demonstrate superiority over other agents,” OPDP said. “Rather, they are intended to show that the effect of a new treatment is not worse than that of an active control by more than a specified margin.” Thus, neither ACUITY-PCI nor REPLACE-2 were acceptable as evidence of Angiomax’s superiority over heparin, the agency said.
Moreover, ACUITY PCI and the HORIZONS AMI study, which TMC also cited, “were confounded by the administration of other anti-thrombin agents prior to the randomized study drugs,” OPDP noted, since almost two-thirds of the patients in both studies were pretreated with enoxaparin or UFH, which “could have contributed to the anticoagulation effect in the Angiomax treatment arms, thus making it difficult to determine the efficacy attributed solely to Angiomax.”
FDA Topples the Pillars of TMC's Angiomax Superiority Claims
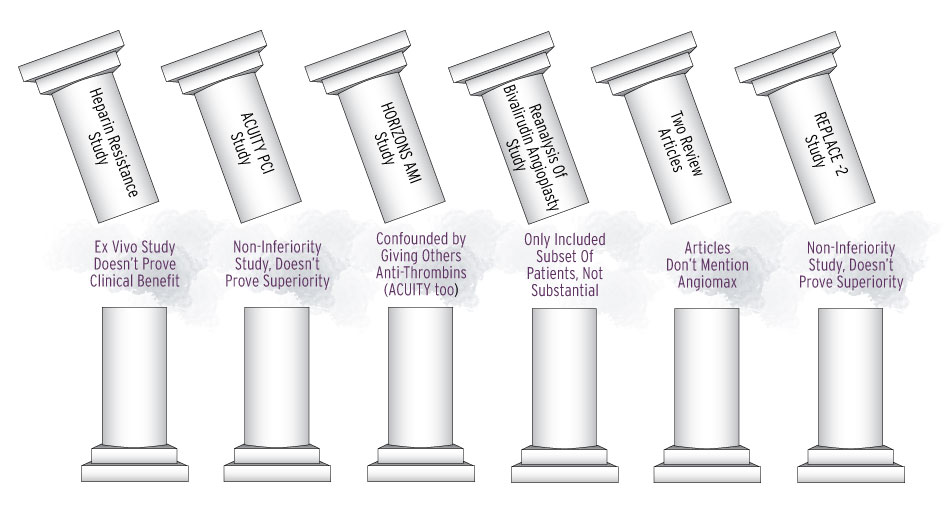
Four other studies TMC cited also failed to make its case for superiority – a study by Rich, et al. that had ex vivo findings, which “do not correlate with claims of clinical benefit implying that heparin has a decreased ability to penetrate the thrombus as the risk of ischemic complications increases”; a reanalysis of the Bivalirudin Angioplasty Trial study that only included patients undergoing percutaneous transluminal coronary (balloon) angioplasty that was irrelevant to TMC’s claims and presentations regarding PCI; and two “review articles [that] do not mention Angiomax at all.”
Thus, OPDP found the booth panel’s claims of “documented victories” “data-driven victories,” “unsurpassed ischemic efficacy throughout the risk spectrum” and “demonstrated unsurpassed ischemic efficacy and reduced bleeding vs. heparin with or without glycoprotein (GP) IIb/IIIa inhibitor” objectionable.
Risk Information Left Out
The panel also left out important risk information for Angiomax, despite including the drug’s contraindications, part of the warnings and precautions regarding bleeding events and some other adverse events.
The panel didn’t include the warning/precaution regarding coronary artery brachytherapy, failed to note that bleeding is the most common AE associated with the drug, seen in 28% of patients, and did not include another warning/precaution regarding the risk of bleeding events, stating that although most bleeding associated with the drug’s in PCI/PTCA occurs at the site of arterial puncture, hemorrhage can occur at any site, and that the drug should be used with caution in patients who have disease states associated with an increased risk of bleeding. Telling viewers to speak to a company representative for more risk information did not make up for these omissions, OPDP said.
TMC is essentially a one-product company, with Angiomax sales of $483.9 million comprising more than 99 percent of 2011 net revenues. The company has been attempting to diversify, however, with a recently announced marketing collaboration deal with AstraZeneca PLC to market the UK-based company’s acute coronary syndromes drug Brilinta (ticagrelor) in the U.S. (Also see "Brennan’s Abrupt Departure Has Some Viewing AstraZeneca As A Buyout Target" - Pink Sheet, 26 Apr, 2012.)