Incyte's Oncology Strategy: Putting Its Eggs In Many Mechanistic Baskets
Executive Summary
Commercial success with Jakafi allows parallel development of many new cancer drug mechanisms beyond the IDO1 inhibitor epacadostat, which is on a fast track.
The financial springboard supplied by the successful JAK inhibitor Jakafi is allowing Incyte Corp. to tackle many new mechanisms all at once, including a second-generation PI3 kinase inhibitor and some of the hottest targets in immuno-oncology today – OX40 and GITR agonists – and rebound from some R&D disappointments.
The company showcased its full pipeline at the American Association for Cancer Research annual meeting in April and is planning on data releases in the second half of the year, perhaps at the European Society for Medical Oncology meeting in October, though during a May 9 earnings call the company said that it’s too early to say what might be included in the program.
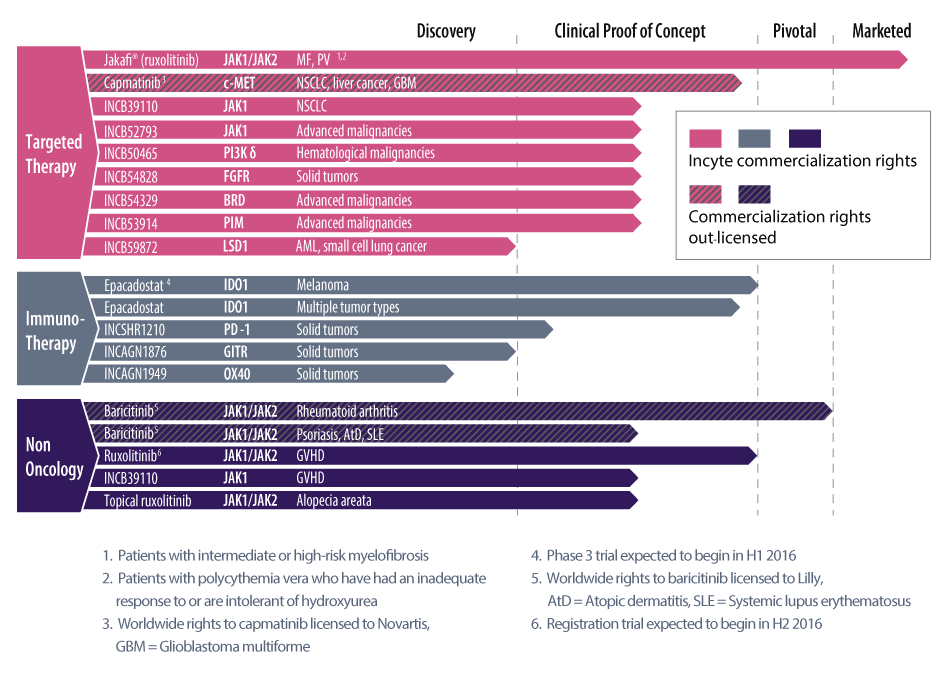
Incyte's pipeline has been undergoing rapid change, execs noted during an investor presentation at AACR, with seven new mechanisms added over the last 24 months. It now has 14 candidates in development, including large and small molecules, spanning 11 different mechanisms.
Whereas the pipeline in the past was heavily weighted toward targeted therapy – a number of programs were related to the JAK family of products – it has been evolving over the last 12 months to give the company a greater stake in immunotherapy, CEO Herve Hoppenot pointed out during a presentation at the AACR meeting.
The IDO1 inhibitor epacadostat was the only immuno-oncology drug in development as of April 2015, but the portfolio now includes three other kinds of immuno-oncology candidates: INCSHR1210, a PD-1 inhibitor licensed from Jiangsu Hengrui Medicine Co. Ltd.; and two preclinical molecules developed with partner Agenus Inc. INCAGN1876, a GITR agonist and INCAGN1949, an OX40 agonist (Also see "Incyte Will Tap Agenus Platform To Move Into Checkpoint Modulator Space" - Pink Sheet, 9 Jan, 2015.). (See chart.)
Incyte Pipeline: April 2016
Incyte notes that it has taken steps to balance and diversify its development portfolio, including large and small molecules.
Source: Incyte investor presentation, April 17
Incyte’s Jakafi (ruxolitinib) has proven very successful with its initial approvals for myelofibrosis and polycythemia vera. But the company has had some setbacks with further development in solid tumors, notably the recent failure in pancreatic cancer and the termination of solid tumor trials investigating the link between inflammation and cancer (Also see "Incyte Ends Jakafi Pancreatic Cancer Trials, But Has More Irons In The Fire" - Pink Sheet, 11 Feb, 2016.). Still, driven by Jakafi, the company’s total revenue rose from $511m in 2014 to $754m in 2015. A pivotal trial of Jakafi in graft-versus-host disease (GVHD) is set to start in the second half.
In its May 9 earnings release, the company reported sales for Jakafi of $183m, up by 59% from the same time last year and increased its full year net revenue guidance from $800m to $815m. The same day, Incyte announced that it was acquiring the European operations of Ariad Pharmaceuticals Inc., including 125 employees, and regional rights to the BCR-ABL inhibitor Iclusig (ponatinib), which is approved for chronic myeloid leukemia and Philadelphia-positive acute lymphoblastic leukemia. The deal includes a $140m upfront payment. The company said that the deal will help maximize the launch success of its own products in Europe. In a May 9 note, Jefferies analyst Brian Abrahams pointed out that Iclusig sales could help offset expenses related to developing wholly-owned assets until new pipeline drugs are approved.
In addition, the rheumatoid arthritis drug baricitinib was recently filed by partner Eli Lilly & Co. and the launch will bring additional royalties to Incyte in the future (Also see "Keeping Track: Lilly Files Baricitinib; FDA Approves New Uses For Novartis' Cosentyx" - Pink Sheet, 25 Jan, 2016.).
For a company of its size, Incyte is in the unique position where it doesn’t need to make trade-offs in its pipeline, rather it is able to move everything forward together in parallel, guided by the science, data and belief in a path forward, Chief Science Officer Reid Huber said in an interview.
To date, epacadostat has become the center of attention in Incyte’s pipeline. Promising mid-stage data combining epacadostat with Merck’s PD-1 inhibitor Keytruda (pembrolizumab) were presented in November at the Society for Melanoma Research annual meeting and a Phase III study of the combination in this indication is set to start in the second half (Also see "New PD-1 Immunotherapy Combinations Push The Envelope In Melanoma" - Pink Sheet, 30 Nov, 2015.).
Epacadostat is in development across 13 tumor histologies in over 900 patients. The development program includes combination studies with the four major sponsors of PD-1/L1 drugs: Bristol, Merck, Roche and AstraZeneca. Data from Phase II expansion cohorts will be released in the second half. The company also is looking forward to the start of enrollment of the Phase III ECHO-301 study of epacadostat with Merck’s PD-1 inhibitor Keytruda (pembrolizumab) for first-line melanoma in the “coming weeks.” Initial data should be released in 2018.
The company will have data from Phase I/II studies for epacadostat in 2016 but declined to comment during its earnings call more specifically in terms of which studies and in what tumor types.
It’s a very broad program but Huber notes that the company is “clearly able to fund that effort and move aggressively into that space without sacrificing in any way what we are doing with other parts of the early development pipeline.”
Leerink Swann analyst Michael Schmidt commented in an April 18 note that while most investors are focused on epacadostat, which is the main value driver in the near term, the “company's early stage pipeline provides several shots on goal and offers diversification long term.”
Beating Zydelig’s Liver Rap
Of Incyte’s 10 presentations at the AACR meeting, one featured clinical data – a Phase I dose escalation trial of INCB50465, the company’s once-daily second-generation PI3 kinase-delta inhibitor, in a range of B-cell malignancies, including follicular lymphoma, diffuse large B-cell lymphoma and chronic lymphocytic leukemia.
The PI3 kinase class has been damaged by experience with Gilead Sciences Inc.’s Zydelig (idelalisib), which was approved by FDA in mid-2014 with a boxed warning for severe liver toxicity and has had low sales since (Also see "The Safety Factor" - Pink Sheet, 20 Oct, 2014.). More recently, new studies of Zydelig were put on clinical hold due to adverse events related to infections when used as part of combinations with commonly used drugs for B-cell malignancies (Also see "Zydelig Takes Major Hit, But Not A Big Blow For Gilead" - Pink Sheet, 14 Mar, 2016.).
As newer members of the class, Incyte’s INCB50465 and TG Therapeutics Inc.'s TG-1202, which is also given once daily, have a structure that has been modified in order to significantly minimize hepatotoxicity side effects.
Furthermore, there are differences in potency. Chief Medical Officer Steven Stein noted that based on published data, INCB50465 is 10 times more potent than the first-generation compounds, both of which are given twice daily – Zydelig and Infinity Pharmaceuticals Inc.’s duvelisib – and 100-fold more potent than TG’s TG-1202.
The response rate in Incyte’s just-reported Phase I study was 60%, including three complete responses, and the disease control rate was 73%
The candidate was well-tolerated over the study period of almost 13 weeks, with no dose-limiting toxicities or need for dose reduction. The rate of Grade 3 and greater adverse events was about 40%, including two cases of anemia, one of bacteremia and one Escherichia infection.
Liver enzyme changes were reported, but did not exceed Grade 1. The company acknowledges that follow-up was relatively short, but on the other hand this effect is usually seen fairly quickly.
The risk for infection seen with Zydelig is likely to be related to the mechanism of action, but it may be possible to treat patients prophylactically to minimize these effects, Huber commented.
“It’s something that gives us some pause, we certainly have to be cautious as we move forward,” he told “The Pink Sheet.”
On top of the adverse event baggage, PI3 kinase inhibitors face a competitive threat from Johnson & Johnson/AbbVie Inc.’s successful BTK inhibitor Imbruvica (ibrutinib), which also is positioned for a range of B-cell malignancies and has demonstrated a fairly benign safety profile.
The targets are very different though, and there may be potential to combine INCB50465 with BTK inhibitors.
“Any decisions we make necessarily have to take into account where that agent is being used and how it is changing the standard of care,” Huber said.
Incyte is planning to start multiple expansion cohorts evaluating INCB50465 as a monotherapy and as part of combination therapy in B-cell malignancies.
A Phase II monotherapy study in relapsed refractory DLBCL is set to start early in the second half of the year. The company has also already moved forward with a Phase II study combining INCB50465 with Jakafi in myelofibrosis as well as proof-of-concept studies evaluating the candidate with the company’s JAK1 inhibitor INCB39110 and Merck’s PD-1 inhibitor Keytruda.
Leerink Swann’s Schmidt said that he was “cautiously optimistic” about INCB50465.
Similarly cautious, JMP Securities analyst Lisa Bayko said in an April 18 note that the “question that remains on investors’ minds is safety," pointing out that "although INCB050465 has structural modifications (removal of the purine group) that seem to dampen liver toxicity, infection risk will remain a class effect.”
Complementary Approaches
Across its pipeline, Incyte is focusing on development in two key areas: immunotherapy and targeted therapies aimed at cell growth and survival signaling, cooperative oncogenic pathways and cancer epigenetics.
In immuno-oncology, PD-1/L1 inhibitors decrease the immunosuppressive nature of the tumor environment, while agonists of GITR and OX40 work on co-stimulatory receptors on T-cells to enhance immune cell activity.
Incyte's Key Development Goals
• Combination of agents that target T-cells directly with agents that modulate the tumor microenvironment may provide optimal anti-tumor immunity.
• Epigenetic mechanisms such as BRD inhibition provide an alternative intervention point for regulating anti-tumor immunity by modulating the tumor microenvironment.
Preclinical data with GITR suggest that antibodies result in rapid and selective elimination of regulatory T-cells in the tumor, Peggy Scherle, VP of preclinical pharmacology, said during the call.
Very similar data have been reported with an OX40 antibody as well, she said. Furthermore, in vivo data suggest enhanced tumor growth inhibition when OX40 is combined with epacadostat.
“Mechanistically, we are still doing additional experiments to explore the mechanistic underpinnings of these results, but the data would support that a greater efficacy will be observed with an agent that targets the T-cell directly in combination with one that targets the tumor microenvironment,” she said.
The company is working with partner Agenus Inc. to move two candidates into the clinic – the GITR agonist INCAGN1876 and the OX40 agonist INCAGN1949 – both of which Scherle said are very selective and “bind very potently to their receptors.”
A clinical study of INCAGN1876 will start within the next several weeks, CFO David Gryska announced during the first-quarter earnings call.
Jefferies analyst Brian Abrahams observed in an April 18 note that the company made a case that its GITR and OX40 antibodies are differentiated in that they have an IgG1 backbone. This feature enables cross-linking which can deplete regulatory T cells selectively, resulting in greater tumor shrinkage in preclinical studies, Abrahams noted.
“How this bears out clinically will help determine how well INCY can catch up with competitive programs further along in development. Synergy with PD-1 inhibitors and/or their IDO inhibitor epacadostat could speak to potential combination approaches down the line,” the analyst said.
Phase I and II OX40 modulators include Glenmark Pharmaceuticals Ltd.’s GBR830 and Bristol-Myers Squibb Co.’sBMS-986178 and AstraZeneca PLC’s MEDI6469, according to the BioMedTracker database. Phase I and Phase II GITR agonists include Bristol’s BMS-986256 Novartis AG’s GWN323 and AstraZeneca’s MEDI1873.
Exploring Epigenetics
The company also played up the potential of its targeted brododomain inhibitor INCB54329 during its AACR presentation.
A brododomain inhibitor led to “profound changes in cytokine levels, both in vitro and within the tumor microenvironment, so that a number of inflammatory cytokines were decreased in a dose-dependent manner,” Scherle said.
The studies are still early, but this suggests “that there is a very strong synergy between epigenetic regulation and immunomodulatory agents,” Scherle said.
The company is currently in Phase I with INCB54329. Huber said that work in the clinic has been focused on hematologic malignancies and tumor-directed therapy.
Incyte also announced during its earnings call that it just dosed the first patient in a study of an LSD1 inhibitor INCB59872, which has an epigenetic mechanism of action. (See chart below for major catalysts in 2016.)
Incyte is starting to get a better appreciation for epigenetics and as “exciting target opportunities emerge there,” it will think hard about them, Huber told “The Pink Sheet”.
For now, the company is “starting to put in place a lot of the tools” needed to develop that unique class of targets, the exec said.
Incyte's Coming Catalysts

* Already completed
Source: Adapted from Incyte's first quarter earnings presentation, May 9, 2016
