How Fast Do PD-1s Get To Market?
Executive Summary
Infographic – FDA set a new record clearing Opdivo for kidney cancer in just under 10 weeks, but the additional indication isn't the only time the agency has picked up the pace for the immune checkpoint inhibitors.
FDA cleared Bristol-Myers Squibb Co.'s Opdivo (nivolumab) for advanced kidney cancer just a week after accepting the supplemental biologics license application (sBLA) for filing – the fastest time yet – but the agency has made a habit of quick clearances for the immune checkpoint inhibitors.
Bristol's CTLA-4 inhibitor Yervoy (ipilimumab) was the first checkpoint inhibitor cleared, and it took the full user fee allotment. FDA acted more quickly with the PD-1 inhibitors, first with Merck & Co. Inc.'s Keytruda (pembrolizumab) and then with Opdivo. Additional indications have been coming fast and furious.
Opdivo's first-to-market kidney cancer clearance on Nov. 23 was the fastest yet, followed by approval in frontline melanoma on Nov. 24 – just a few days short of the user fee deadline.
Immune Checkpoint Review Times, In Days
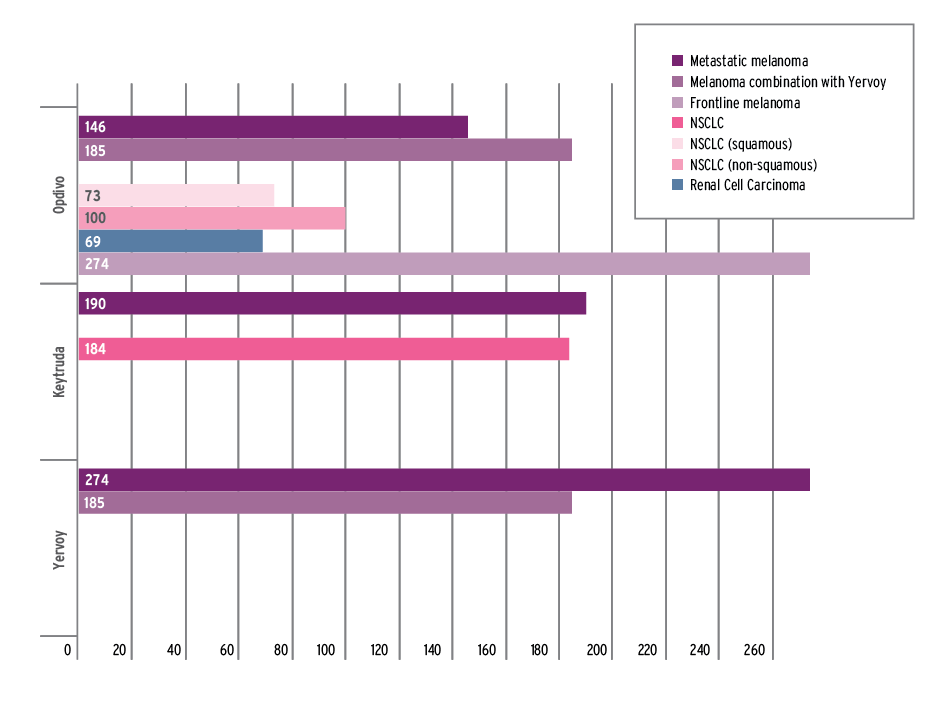
FDA has recognized the tremendous medical advances represented by the immune checkpoint inhibitors, working with sponsors to get the cancer immunotherapy agents to market quickly through programs like breakthrough designations and accelerated approval. The Office of Hematology and Oncology Products regularly beats review deadlines, but the times achieved across the programmed-death inhibitor class have been unprecedented. With more offerings to come, FDA is likely to continue to make the regulatory review the fastest part of the development process.
To track pending applications and breakthrough designations, visit the FDA Performance Tracker![]() .
.
