Six Ways For PD-1 Lung Cancer Programs To Differentiate
Executive Summary
The PD-1 and PD-L1 inhibitors have delivered stellar data in a number of tumor types and hold mega-blockbuster promise – but with so many in the class, sponsors are looking to stand out. Pull-out table provides side-by-side comparison of leading programs.
With the PD-1 and PD-L1 programs looking striking but similar, differentiation in the prized lung cancer indication may lie in the timing and structure of development programs.
Lung cancer always has been the most eagerly awaited indication for cancer immunotherapy, and the vanguard of PD-1/L1 checkpoint inhibitors. It was the next target behind the lead indication of melanoma and the initial data have not disappointed, as data presented at the recent American Association for Cancer Research meeting showed (Also see "PD-1 Data Promise More Shake-ups In Melanoma Standard of Care" - Pink Sheet, 20 Apr, 2015.).
In some ways Bristol-Myers Squibb Co. already has won the race, with its Opdivo (nivolumab) clearing FDA in March for squamous type NSCLC, regardless of PD-L1 expression (Also see "Opdivo Lung Cancer Approval Exceeds Rosiest Expectations" - Pink Sheet, 4 Mar, 2015.). But Merck & Co. Inc. is close behind with a recent filing of Keytruda (pembrolizumab) for accelerated approval, based on results from the single-arm KEYNOTE-001 study, in which the drug demonstrated dramatically better response rates in a PD-L1 selected population.
The role of the PD-L1 biomarker and the settings and subpopulations will be the early battleground for the market, and Bristol and Merck soon will be joined by oncology heavyweight Roche and AstraZeneca PLC, hoping to come from behind.
During its first-quarter earnings call on April 22, Roche said the base case for filing its PD-L1 inhibitor MPDL3280A is in 2016 but it has a number of potentially registrational Phase II studies ongoing that could result in filing in 2015. Chief Operating Officer Daniel O’Day told analysts: “We will continue to look for ways to bring that forward, I can assure you.”
AstraZeneca is still hoping for an opportunity to file its PD-L1 inhibitor MEDI4736 in PD-L1 selected third-line NSCLC patients by the end of the year.
FDA has issued multiple “breakthrough” designations for the class and quickly approved any programmed-death application filing. The challenge for the sponsors lies in that they will all have very good drugs.
An Equal Opportunity Revolution
The members of the PD-1/L1 class look very similar, though there may be some “subtle differences,” Johns Hopkins’ Leisha Emens, lead investigator on a study of Roche’s MPDL3280A in triple-negative breast cancer, said during a briefing at the recent American Association for Cancer Research annual meeting.
Commenting in an April 19 blog![]() post about the encouraging AACR news in his field, Seattle Swedish Cancer Institute lung cancer specialist Howard Jack West asked whether it’s time to ask whether these agents really provide meaningful differences if given to the same patients, or instead whether there would just be differences in marketing for “remarkably similar treatments.”
post about the encouraging AACR news in his field, Seattle Swedish Cancer Institute lung cancer specialist Howard Jack West asked whether it’s time to ask whether these agents really provide meaningful differences if given to the same patients, or instead whether there would just be differences in marketing for “remarkably similar treatments.”
“Are we really just seeing results for Coke and Pepsi, perhaps with a newly created ‘Pepsi Generation’?” the clinician asked.
“I think there’s good reason to suspect that Coke and Pepsi are more distinctive than these two agents with the same mechanism of action,” he added.
Merck’s Roy Baynes, senior VP of global clinical development, commented in an interview that PD-1 development is not necessarily a “winner-takes-all arena,” rather there will be “significant opportunities for a number of groups.”
All of the sponsors faced questioning about their competitive strategy in the PD-1 space during the first quarter earnings calls (Also see "PD-1 Round-Up: Big Pharmas Talk Competitive Positioning" - Pink Sheet, 4 May, 2015.).
Differentiation, Baynes said, comes largely from the development programs and depth of data.
Interviews with sponsors, analyst research and a review of the pipeline suggest a range of areas for differentiation, including:
- The size of the program,
- Speed to market,
- Simplicity,
- Biomarkers,
- Niche sections of the market, and
- Combinations.
How The Programs Stack Up
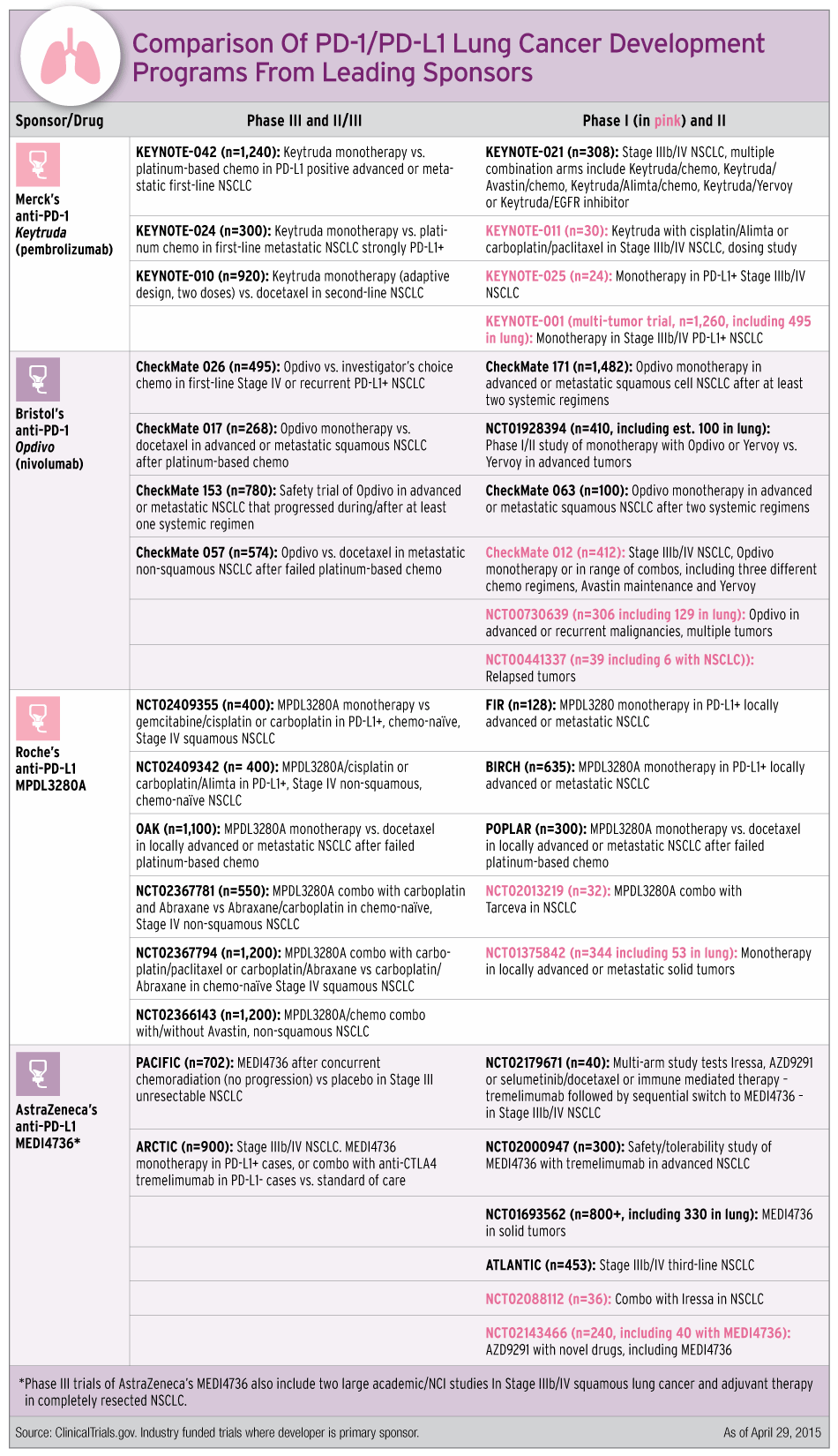
While many aspects of the PD-1/L1 development programs are similar, they are designed with some differentiation in mind. A number of the Phase I and II studies listed above potentially could be registrational.
Size Matters
Perhaps the crudest way to compare programs is simply to look at the size. And by that gauge, including recently announced studies, Roche has the biggest Phase III development program, with six trials in 3,650 patients. Competitors faced questions during first-quarter earnings calls about Roche’s “aggressive” Phase III program.
Bristol and Merck were on par as of late April, though Bristol is planning to start a new study soon of its CTLA-4 inhibitor Yervoy (ipilimumab) in combination with Opdivo in first-line lung cancer.
AstraZeneca, the latest to join the PD-1 race, has the smallest program with two Phase III trials in a total of about 1,600 patients, but its Phase II ATLANTIC study is potentially registrational and its MEDI4736 is part of NIH’s Lung-MAP trial (Also see "Lung-MAP Patient Screening Should Translate To Cost-Savings" - Pink Sheet, 23 Jun, 2014.).
AstraZeneca has been focusing on niche opportunities in both early and late-line treatment of NSCLC. The company’s Phase III PACIFIC study tests MEDI4736 after chemoradiation in Stage III unresectable NSCLC. The company has noted that it was the first to conduct a trial in the adjuvant setting, through a partnership with the National Cancer Institute of Canada (NCIC) Clinical Trials Group (Also see "With Oncology Drugs On The Way, AstraZeneca Sets The Stage For New Launches" - Pink Sheet, 26 Jan, 2015.). Looking at late-line therapy, AstraZeneca’s Phase II ARCTIC study in third-line could allow a filing for accelerated approval in 2015.
Bristol notes that about 80% of lung cancers are metastatic at the time of diagnosis, but that it too will be looking to take Opdivo into earlier lines of therapy.
|
Size of PD-1/PD-L1 Lung Cancer Development Programs |
||
|
Drug |
Phase III |
Total Clinical Program |
|
Merck’s Keytruda |
Three trials, 2,460 |
3,317 |
|
Bristol’s Opdivo |
Four trials, 2,629 |
5,185 |
|
Roche’s MPDL3280A |
Six trials, 4,850 |
5,998 |
|
AstraZeneca’s MEDI4736 |
Two trials, 1,602 |
2,801 |
|
Source: Calculated from ClinicalTrials.gov listings of trials (as of May 5) where primary sponsor is PD-1/L1 developer. For multi-tumor trials, includes lung patients only. |
||
Keep It Simple
Bristol and Roche both are testing and planning to introduce a single dose and schedule in all late-stage studies across all tumor types, including monotherapy and combination regimens.
“A consistent fixed dose across trials may help us to best understand the safety and efficacy of our medicine,” Roche explained.
Merck is testing multiple doses and AstraZeneca says it hasn’t determined yet whether there will be standardized dosing.
Embrace Biomarkers
PD-L1 expression has emerged as the leading biomarker to date for PD-1/L1 inhibitors, but its use has been subject to endless debate – which was refueled after the latest round of lung cancer data at AACR (Also see "Latest PD-L1 Lung Cancer Data Increases Debate On Biomarker" - Pink Sheet, 27 Apr, 2015.). Patients testing negative for the biomarker can still respond to treatment, while biomarker status changes over time.
All the sponsors have built stratification into their trials to test both in high-expressers and in all-comers, and some have run dedicated trials in over-expressers. Merck and Roche have been most committed to the biomarker.
Merck’s KEYNOTE-001 study, the basis of the lung cancer filing, showed significantly higher response in the PD-L1+ population than in the total population, and Dako Corp. submitted a tandem application for a companion diagnostic.
During its April 28 first quarter earnings call, Merck executives noted the biomarker is expected to be particularly useful for physicians and payers who want to develop treatment strategies and algorithms for use of the drugs.
Bristol, however, has approval for the broad population (not tied to PD-L1 expression). Fouad Namouni, global development lead for nivolumab, has said that the science suggests not preselecting patients with a biomarker, but rather looking at the results for biomarker-negative and -positive patients and drawing conclusions when you see data for the right endpoint.
Roche, which is developing its own companion diagnostic, believes that the quality of testing matters a lot and that data strongly support the biomarker. The company asserts there has been a consistent signal for PD-L1 expression across its program.
Evaluation of PD-L1-selected patients allows a route for early approval based on objective response rates. However, there are questions about whether companies with overall survival will have a competitive advantage or alternatively whether the drugs will be viewed as interchangeable and the data therefore not as important.
Speed Is Of The Essence
Speed of development and time-to-market are crucial. Morningstar analyst Damien Conover, who has been bullish on the potential for PD-1 inhibitors, particularly in lung cancer, stressed the importance of the first-mover advantage in a comprehensive February 2015 report on immuno-oncology (see box).
PD-1 Projections In Lung Cancer
- Non-small cell lung cancer represents 85% of lung cancers
- About 400,000 people worldwide use drug treatment for NSCLC, including all lines, but survival rates still leave a lot of room for improvement and need for new drugs
- Massive support for PD-1 and PD-L1 development is shown by the over 17,000 patients in studies for these drugs
- Lung cancer pricing likely will be on par with melanoma – $140,000 per year – but for a longer duration
- Immuno-oncology drugs likely to drive $25 billion lung cancer market
- Morningstar projects sales of $8 billion and $6 billion for Opdivo and Keytruda in lung cancer in 2020, above consensus of $6 billion and $3 billion, respectively
Source: Damien Conover, Morningstar Research
“Over the past decade, cancer drugs with similar to slightly better efficacy and similar mechanisms of action to already approved drugs only gained 2%-10% market share after three years on the market. The first-mover advantage in the cancer market has several drivers, including better label expansion opportunities, the availability of long-term data for the first mover, and a higher bar for trial design for new therapies,” the analyst noted.
“While all four leading companies (Bristol, Merck, Roche and AstraZeneca) will share in the first wave of approvals in immuno-oncology,” Bristol has the strongest first-mover advantage, Conover said, referring to the 2011 approval of Bristol’s Yervoy (ipilimumab), the first checkpoint inhibitor on the market.
With the first-to-market advantage overall, Merck’s Keytruda brought in $83 million in the first quarter, while Bristol’s Opdivo lagged behind with $40 million for the first quarter (Also see "Bristol Says It’s Ready For PD-1 Competition In Lung Cancer" - Pink Sheet, 28 Apr, 2015.). The true test of Opdivo being first with a lung cancer approval, which came March 4, will be next quarter. “The early sales and data are coming in largely as we projected,” Conover told “The Pink Sheet.”
Bristol is counting on the strength of its data in lung cancer to keep it in a preferred position with payers. Labeling already shows a survival benefit in squamous NSCLC, and the firm soon will submit survival data from the recently halted CheckMate 057 study in the larger population of non-squamous NSCLC. Based on the FDA approval timelines in squamous disease, approval in the third or fourth quarter of 2015 can be expected, Mirabaud Securities analyst Nick Turner commented in an April 21 note.
Merck, however, initially will have only the support of response rate data from a single-arm trial, which underscores the importance for Merck in releasing results from the randomized KEYNOTE-010 trial, a second-line study testing Keytruda against chemotherapy. UBS Equities analyst Alexandra Hauber said that the CheckMate 057 data “should make it harder to argue [there is] unmet medical need” not addressed by single agent PD-1/L1 inhibitors.
Find Your Niche
AstraZeneca suffered from unflattering comparisons to its rivals in terms of speed to market after the AACR meeting. But it acknowledges that it was at the back of the pack, and has developed its strategy accordingly. At the American Society of Clinical Oncology meeting in mid-2014, the company said that it planned to lead from behind by finding unique sections of the market (Also see "AstraZeneca Playing Leapfrog In Immunotherapy: How It Plans To Lead From Behind" - Pink Sheet, 9 Jun, 2014.).
While the initial approvals and submissions have been in the second- or third-line settings, there is still an opportunity to move to first-line use.
Bristol’s early approval was in squamous cell disease, but it is readying a submission of its non-squamous trial CheckMate 057. Merck’s pivotal KEYNOTE-001 is in squamous and non-squamous patients.
AstraZeneca stresses that it is the only company with a Phase III adjuvant trial ongoing and the only one with a registrational trial in locally advanced, unresectable disease – a segment it prioritized after identifying it as an untapped area (Also see "Q&A: AstraZeneca Execs Talk Immunotherapy Strategy And More At ASCO" - Pink Sheet, 9 Jun, 2014.).
“Both represent significant segments of patients, and we have the potential to be first in both,” Chief Medical Officer Briggs Morrison told the firm’s April 24 earnings call.
It also has a fast-to-market strategy for the third-line setting with the ATLANTIC study, for patients who over-express PD-L1, but its shot at accelerated approval may be blocked if Merck gains full approval for the PD-L1+ population. “As of today, we believe this opportunity is indeed viable and we are focused on getting the data and potentially preparing a submission as quickly as possible,” Morrison said.
However, analysts may not buy in to its strategy. “AZN is behind the competition from BMY, MRK and ROG but had revealed a fast to market strategy it was confident could leap-frog the other players in high value indications, particularly NSCLC and head and neck cancer. It looks very much as if it may have been outflanked,” Mirabaud’s Turner said.
It is debatable how much the specifics of approvals and datasets will matter once a drug is on the market. Oncologists are accustomed to working from evidence and compendia without waiting for regulatory approval. Morningstar’s Conover pointed out that approval opens the door for use across stages and indications and can increase physician confidence due to the availability of longer term data.
Roche’s niche might lie in developing a wide array of combinations.
Combination Strategy, And A Combination Of Strategies
All of the PD-1/L1 sponsors view combination strategy as a hugely important part of development. Bristol and AstraZeneca both have CTLA-4 inhibitors (Yervoy and tremelimumab, respectively) that are being tested in combination with PD-1/L1 inhibitors for a double immunotherapy approach. New types of immunotherapies earlier in development also could prove to be good partners.
Bristol’s current Phase III program tests Opdivo as a monotherapy head-to-head against chemo, but the company is planning to start a first-line NSCLC study of Yervoy with Opdivo in all comers, regardless of PD-L1 expression, soon. The combination has shown strong efficacy, but the high rates of severe adverse events disappointed oncologists at the 2014 ASCO meeting.
However, if the company can demonstrate a significant improvement in survival, that could trump concerns about toxicity. The release of results for the Opdivo/Yervoy combination in lung cancer and melanoma at the upcoming ASCO meeting May 29-June 2 could prove to be a turning point.
AstraZeneca has a Phase III study up-and-running called ARCTIC that is evaluating its PD-L1 inhibitor MEDI4736 in combination with tremelimumab for PD-L1-negative NSCLC and will be providing an update at the ASCO meeting about early data for the combo.
To date, it’s been hard to differentiate the PD-1 from PD-L1 inhibitors on either efficacy or safety. Sponsors of PD-L1 drugs – AstraZeneca and Roche – have been looking to see a benefit for safety due to a small difference in the mechanism of action. Now, they still have hope that the tolerability differences could emerge in combination studies.
AstraZeneca has said that even small differences in rates of toxicity could make a difference in earlier disease settings as well as in combinations, whether with chemotherapy or targeted small molecules or other types of immunomodulators (Also see "With Oncology Drugs On The Way, AstraZeneca Sets The Stage For New Launches" - Pink Sheet, 26 Jan, 2015.).
Early-stage studies have suggested feasibility for combining PD-L1 inhibitors safely with chemotherapy. Those data encouraged the company to move quickly and strongly into front-line NSCLC studies combining MPDL3280A in combination with chemotherapy, Dan Chen, Genentech’s cancer immunotherapy franchise head, said.
Roche recently announced a range of new Phase III studies. The company’s late-stage program in lung cancer now includes four studies testing MPDL3280A in combination with a variety of chemotherapy regimens.
“Chemo is very active in frontline NSCLC and is the standard of care, so the ability to add PD-L1, to inhibit PD-L1 and get either additive or synergistic activity will go a long way we believe to helping people with non-small cell lung cancer,” Chen said.
Roche will be presenting Phase I data for MPDL3280 in combination with chemotherapy at the ASCO meeting.
In addition to established drugs, sponsors are developing a range of investigational drugs, including new immunotherapies, that could be used in combination with the PD-1 class. Some are being developed in-house and others are being tested through deals.
But a sound combination strategy alone will not be enough to claim the biggest share of the market – a combination of strategies will be needed.
“The winning companies will control the broadest combo therapy portfolios and enter markets first,” Conover said in his immuno-oncology report.
