CLL Market Update: Imbruvica Leads Way, Jump-Started By Robust Launch
Executive Summary
Recent BioMedTracker surveys suggest that most doctors are using new oral drugs – J&J/Pharmacyclics Imbruvica and Gilead’s Zydelig – generally in line with labeling, while uptake of Roche’s Rituxan-successor Gazyva is lower than expected.
Johnson & Johnson/Pharmacyclics Inc.’ oral Imbruvica has emerged on top in the shakeup of the treatment paradigm for chronic lymphocytic leukemia, with strong sales and positive sentiment from prescribing doctors at the close of 2014.
The markets for treating CLL and the related condition small lymphocytic lymphoma (SLL), as well as other B-cell malignancies, have been poised for transformation following the introduction of three new drugs since November 2013: Imbruvica (ibrutinib), which is a BTK inhibitor, Gilead Sciences Inc.’s oral PI3 kinase inhibitor Zydelig (idelalisib) and Roche’s anti-CD20 antibody and Rituxan (rituximab)-successor Gazyva (obinutuzumab) (Also see "CLL Market Snapshot: Seismic Changes Rock The Treatment Landscape" - Pink Sheet, 20 Oct, 2014.).
Imbruvica has gained the lead, bringing in approximately $492 million in U.S. product sales in 2014, including $185 million in the fourth quarter, far outstripping the full-year performance for Gazyva, with CHF 49 million ($53 million), and Zydelig, at $23 million. On top of the $492 million, Imbruvica had an additional $56 million in sales outside the U.S., where the product is managed by J&J.
Survey Says...
In a February survey on usage and reimbursement by BioMedTracker, 95% of 40 responding hematology/oncology specialists respondents said that within the last three months they prescribed Imbruvica to some degree, while 70% were writing scripts for Zydelig – though 80% reported writing more scripts for Imbruvica than for Zydelig. Physicians reported high rates of reimbursement for labeled indications of both drugs.
BioMedTracker’s survey indicated that the most common kind of insurance for CLL was Medicare, followed by private insurance. In the survey, 47.5% said that Imbruvica was often covered by insurers for first-line treatment of CLL patients with 17p deletions and 20% said it was usually or always covered for this indication. Responses were considerably lower for first-line treatment without the deletions (not an approved indication) at 27.5% and 15% respectively. In relapsed/refractory CLL (all types), 40% reported that treatment was often covered and 42.5% said it was usually or always covered.
The three recent additions to the CLL market have slightly different indications, and Imbruvica has the widest variety of claims. Imbruvica was approved for relapsed/refractory CLL in February 2014, following clearance in mantle cell lymphoma in November 2013. In July 2014, the drug picked up an indication for frontline treatment of CLL in patients with the hard-to-treat 17p deletions. On Jan. 29, the drug added yet another indication, for Waldenstrom’s macroglobulinemia, a rare kind of non-Hodgkin lymphoma that affects only 1,500 patients in the U.S. each year.
During Pharmacyclics’s Jan. 12 presentation at the J.P. Morgan Healthcare Conference in San Francisco, CEO Bob Duggan noted that Imbruvica would be the only approved agent for this condition and added that while the patient population is small, the duration of treatment is long, at from three to five years.
When Pharmacyclics’ fourth quarter/full year earnings were released on Feb. 18, Chief Commercial Officer Shawn Tomassello said that revenue hasn’t been broken out by type of patient (for example, those with 17p deletions) but that the company “continues to see an increase in its market share position across all lines.”
At J.P. Morgan, Duggan had labeled Imbruvica the “fastest-growing medicine in the history of the hematological world,” pointing out that the launch performance compares favorably to other successful oncologics.
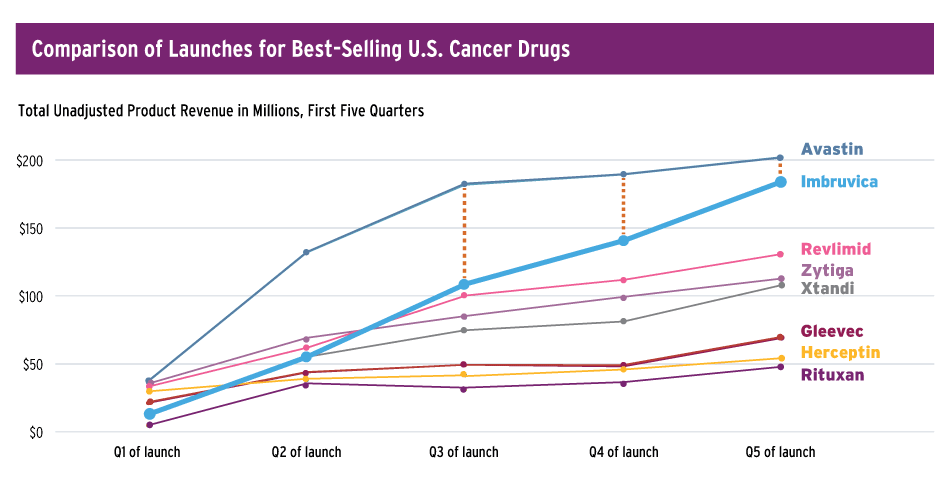
Pharmacyclics says that performance of its BTK inhibitor Imbruvica compares well to other cancer drug launches.
Source: Pharmacyclics
The drug’s U.S. revenue of $492 million for 2014 exceeded all expectations and the company closed the year with $850 million in cash, Duggan noted. Looking ahead, the company anticipates “doing $1 billion in revenue this year, representing a 103% increase over the expected 2014 U.S. product revenue,” he added.
Now the company is driving expansion in hematology, with eyes on many indications that create a total target global population in hematology indications of 374,000 (see chart), but it also will be developing the drug in solid tumors and immunological diseases outside of oncology. Pharmacyclics has 13 Phase III clinical studies of Imbruvica running and overall, including investigator-sponsored and company studies, 58 trials are ongoing and an additional 82 are in progress.
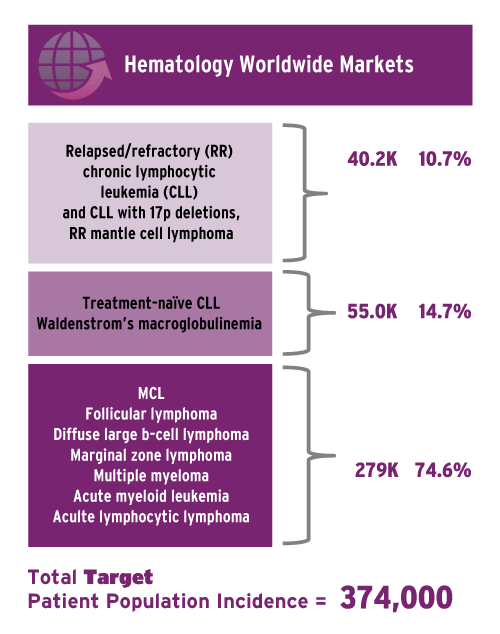
Source: Pharmacyclics
“Our minimum goal is adding one to two new labels per year,” Duggan said.
Duggan stressed the long duration of therapy during the company’s J.P. Morgan presentation, noting that the drug’s “safety profile allows for long-term dosing and restoration of hematological function.” He pointed out that the longest patient on study had received the drug for five years and two months.
However, in a February 2015 CLL market report, BioMedTracker analysts noted that bleeding-related adverse events with Imbruvica are an important factor for consideration by physicians. FDA labeling notes the potential risk for hemorrhage – including fatal events – in patients taking anticoagulants and antiplatelet drugs (Also see "The Safety Factor" - Pink Sheet, 20 Oct, 2014.). The “mechanism for the bleeding events is not well understood,” according to the label, and while it is not contraindicated for patients on blood thinners, in practice this may be the case.
“Considering the CLL patient population (elderly patients), this contraindicates ibrutinib in many patients with atrial fibrillation or at risk for thrombosis. These patients may receive idelalisib – or, in the future, other approved agents – rather than ibrutinib,” the BioMedTracker report says.
Pharmacyclics has previously stressed that the bleeding events were mostly low-grade effects and is studying the risk as a post-marketing commitment to FDA.
Gazyva’s Slow Start
At CHF 49 million ($53 million), 2014 sales for Roche’s Gazyva were very modest, especially compared to Imbruvica’s meteoric rise in the market.
Gazyva was approved in the U.S. in November 2013 for use with chlorambucil chemotherapy in frontline CLL (Also see "Roche’s Gazyva Clears FDA, But First Breakthrough Approval Breaks No Speed Barriers" - Pink Sheet, 4 Nov, 2013.). The drug was approved for frontline CLL in Europe in July 2014 and was recommended for coverage by the U.K.'s National Institute for Health and Care Excellence at the end of the year (Also see "EMA Endorses Two Orphans: Roche’s Gazyvaro and PTC’s Translarna" - Pink Sheet, 23 May, 2014.). Most of the product sales derived from the U.S. market.
Results from a BioMedTracker survey in December 2013 suggested robust uptake of Gazyva to come, but in the end this did not translate into sales. Whereas 44% of prescribers had indicated in December 2013 that they would prescribe Gazyva to at least 25% of patients, in a January 2015 follow-up survey by BioMedTracker, only 9% of physicians said their usage was above this level.
In a recent BioMedTracker survey, 60% of physician respondents were prescribing Gazyva to at least some patients, but most were doing so at low levels.
While 60% of respondents were prescribing Gazyva to at least some patients, the drug was prescribed at “low levels by the majority of physicians,” BioMedTracker analysts reported.
The company has suggested that one factor behind the slow launch was the type of combination used in the pivotal CLL-11 study that supported approval. The three-arm study evaluated Gazyva with chlorambucil against Rituxan/chlorambucil and chlorambucil alone in first-line treatment of elderly patients. Chlorambucil is a weaker kind of chemotherapy that is less commonly used in the U.S. relative to Europe. The company reported safety results in December 2014 for Gazyva with more commonly used chemotherapies in the GREEN study.
On Dec. 24, 2014, FDA approved a supplemental BLA expanding labeling to include many additional data points from the CLL-11 study (Also see "A Busy Year-End, In Brief: December Brings Slate Of Approvals, And Setbacks" - Pink Sheet, 2 Jan, 2015.). For example, labeling now notes that people taking Gazyva with chlorambucil lived longer without disease worsening than those taking Rituxan/chlorambucil and that the complete response rate was significantly better (26.1% versus 8.8%) with Gazyva, as were the results from the perspective of rates of minimal residual disease following treatment.
In the CLL-11 study, Gazyva/chlorambucil demonstrated a survival benefit against chlorambucil alone, but not against the Rituxan combination arm. FDA labeling indicates that the overall survival data for Gazyva/chlorambucil vs. Rituxan chlorambucil are not yet mature.
BioMedTracker analysts believe the lack of an overall survival benefit compared to Rituxan combination therapy is a drawback. And while the Gazyva/chlorambucil regimen was most effective in terms of progression-free survival and response rates, “it’s also associated with high rates of infusion-related reactions,” including severe events (see table).
Good performance in combination studies could help Gazyva gain more market share in the future (see related story, (Also see "CLL Sponsors Look To Create Winning Combinations" - Pink Sheet, 23 Feb, 2015.)).
|
CLL-11 Study Results |
||||
|
Study stage |
Stage 1 |
Stage 2 |
||
|
Treatment |
Gazyva/chlorambucil |
Chlorambucil |
Gazyva/chlorambucil |
Rituxan/chlorambucil |
|
Median PFS |
27.2 months |
11.2 months |
26.7 months |
14.9 months |
|
Median OS |
Combination of Gazyva/chlorambucil reduces risk of death by 59% compared to chlorambucil alone. OS Results for Gazyva/chlorambucil vs. Rituxan/chlorambucil are not yet mature. |
|||
|
Response Rates |
Overall 78.2%, 28.2% complete response (CR) |
33.1% ORR, 0 CR |
79.6% ORR, 26.1% CR |
66.3% ORR, 8.8% CR |
|
Select adverse events |
Neutropenia: 41% overall and 35% Grade 3-4; infusion reactions 69% overall, 21% Grade 3-4 |
Neutropenia: 18% overall, Grade 3-4 16%; no infusion reactions of any grade |
Neutropenia overall 38%, Grade 3-4 33%; infusion reactions, 66% overall and 20% Grade 3-4 |
Neutropenia 32% overall, Grade 3-4 28%; infusion reactions 38% overall and 4% Grade 3-4 |
|
Source: Product labeling |
||||
Zydelig Lagged As Well
Safety issues may also help explain the lackluster performance of Zydelig in 2014, in addition to its later approval relative to Imbruvica and Gazyva. Zydelig was approved by FDA in July, five months after Imbruvica’s clearance for the CLL indication and seven months later than Gazyva’s approval. Gilead’s drug was approved as a relapse therapy in combination with Rituxan for CLL, SLL and follicular lymphoma (Also see "Gilead Doesn’t Push Limits In Pricing Its New Lymphoma Drug Zydelig" - Pink Sheet, 23 Jul, 2014.).
The drug – which is Gilead’s first oncology product – had just $16 million in U.S. sales for 2014. In Europe, where the drug was approved for FL and CLL in September 2014, sales reached $7 million; the company told its year-end earnings call on Feb. 3 that the “pricing and reimbursement process is ongoing.”
Zydelig labeling includes a boxed warning for serious (potentially fatal) hepatotoxicity, which occurred in 14% of patients in the clinical trial safety population, serious diarrhea or colitis, serious intestinal perforation, and serious pneumonitis.
“Although idelalisib is effective, its safety profile has perhaps put a damper on uptake, especially since ibrutinib launched first,” the BioMedTracker CLL market report states.
Other PI3K inhibitors are in development and theoretically could be differentiated on safety.
For example, Infinity Pharmaceuticals Inc. and partner AbbVie Inc. are developing duvelisib, formerly called IPI-145 (Also see "Infinity Gets More Than A Cash Boost With AbbVie Tie-Up" - Pink Sheet, 3 Sep, 2014.). Duvelisib inhibits the delta and gamma isoforms of the p110 catalytic subunit of PI3K and it is unclear whether it will have the same adverse event profile as the delta-only inhibitor idelalisib, BioMedTracker analysts note.
“It is possible that the isoform specificities of other PI3K-family inhibitors will lead to different safety profiles, but these agents will need to pass through larger registrational trials to verify early safety results,” the CLL market report states.
In BioMedTracker’s February reimbursement survey, 47.5% of hematologist/oncologist respondents said that Zydelig was often covered for CLL patients after at least one prior therapy and 32.5% said that it was usually or always covered for this indication. As for off-label use as a first-line treatment in CLL patients with 17p deletion, 27.5% said that the drug was often covered versus only 5% who reported it’s usually or always covered for this indication.
Embracing Change
Whereas Gazyva’s treatment path has already been established, as it is intended as a replacement for fellow anti-CD20 antibody Rituxan, Imbruvica and Zydelig are both oral drugs given until disease progression. That represents a big change from past treatment patterns, given that the disease traditionally was treated with chemotherapy and/or an anti-CD20 antibody for a fixed period.
There had been some uncertainty about how quickly physician prescribers would be willing to shift to a new treatment paradigm, but judging by Imbruvica sales, physicians have embraced the change.
Results from a BioMedTracker survey of 22 specialists in January suggest that Imbruvica was most commonly being prescribed as a first-line treatment for those with 17p deletions – 54.5% of respondents said that they would often prescribe for this indication and 22.7% said that they would always prescribe Imbruvica in first-line 17p deletion patients.
There has been some speculation about whether the drug would be used off-label. In the survey, 40.9% said they would rarely prescribe Imbruvica for off-label use as a first-line treatment to patients without 17p deletions, and 27.3% said they would often prescribe for this indication.
Compared to Imbruvica, Zydelig labeling is more restrictive. Gilead’s PI3 kinase inhibitor is FDA-approved for use with Rituxan in relapsed CLL in cases where Rituxan alone would be considered appropriate due to comorbidities, which is a later line of relapse therapy compared to Imbruvica, which is approved for use after one prior treatment.
In BioMedTracker’s February survey, 40.9% said that they would prescribe Zydelig rarely for first-line treatment in patients without 17p deletions and 13.6% said they would often prescribe it for this indication.
When it came to forecasting plans for future use, doctors indicated plans to use Imbruvica more than Zydelig across indications. However, overall, 68% of respondents said that they planned to use both Imbruvica and Zydelig often or always.
The survey also presented physicians with early trial data for Roche’s investigational oral BCL-2 inhibitor venetoclax (ABT-199) in relapsed/refractory CLL. Results indicated that if approved, this drug would be used at a similar level as Imbruvica and Zydelig, but lead author Jolene Lau concluded that Phase III data “will strongly affect eventual uptake,” particularly data on duration of response and side effects.
