FDA To Close Office In Amman, Jordan; Operations Moving To White Oak
Executive Summary
Opened as part of the globalization initiative, FDA’s presence in the Middle East and North Africa will shut down in late December in an apparent cost-cutting effort.
FDA will close one of the foreign offices it has opened in recent years to help deal with the globalization of the supply chain, a move that appears to have hinged on saving precious funding.
The agency office in Amman, Jordan, which was responsible for helping with drug and other facility inspections and providing training and information about agency regulations in the Middle East and North Africa, will close Dec. 25. FDA then plans to base work in the region at its White Oak headquarters in Silver Spring, Md.
The agency said in a Sept. 19 email that it is looking for continued “engagement in scientific and regulatory collaboration” in the Middle East and North Africa (MENA), but decided resources were best used with the post located in the U.S.
“After considering the position and its resource environment overall, the agency believes that it could best leverage FDA resources and that of other agencies and countries in the MENA region by moving the position back to FDA headquarters,” the agency said.
It seems FDA was forced to make a decision that many pharma companies have faced: whether to close a location that may no longer be cost-effective in an environment where money is scarce.
FDA had $209 million in user fees and treasury dollars taken from its FY 2013 budget when the sequester went into effect. Its budget also has not grown much in recent years as members of Congress have attempted to cut spending across all federal departments (Also see "FDA Budget May Need To Be Trimmed 5% In FY 2015" - Pink Sheet, 31 May, 2013.).
The agency received an additional $10 million in fiscal year 2013 appropriations to be used to increase its inspections and training in China (Also see "FDA Highlights Hands-On Approach To China Inspections In Budget Plan" - Pink Sheet, 27 Feb, 2012.). But the visas for a number of the additional employees have not been issued by the Chinese government.
The Amman office had a staff of two people, including Senior Regional Advisor Layla Batarseh, who was FDA’s primary representative in the region. The agency said inspections in the area will continue consistent with policies and procedures.
“FDA remains committed to engaging with its partners in the MENA region, including regulatory counterparts, industry, multilateral organizations, academic institutions and other U.S. government agencies,” the agency said in its statement.
Raphael Brykman, acting team leader in the FDA Office of the Commissioner’s Office of Strategic Partnerships and Analytics, said Sept. 17 those working in the office would be returning to the U.S.
Brykman also said FDA has not decided to pull its physical presence from the region forever.
“It might be relocated somewhere else in the future,” Brykman said during a presentation at the Parenteral Drug Association-FDA Joint Regulatory Conference. “We’re not sure yet.”
FDA has a number of foreign offices around the world, including in Chile, China, Costa Rica, India, Mexico, South Africa and multiple locations in Europe (see table). They are intended in part to allow the agency to conduct more foreign inspections quicker. The first overseas office opened in 2008 in Beijing, China (Also see "FDA Launches First Overseas Office In Beijing" - Pink Sheet, 20 Nov, 2008.).
The Amman office will join the Asia-Pacific office, which is responsible for countries like Australia, Canada, Japan and South Korea, in operating out of White Oak.
Responsibilities of FDA Foreign Offices
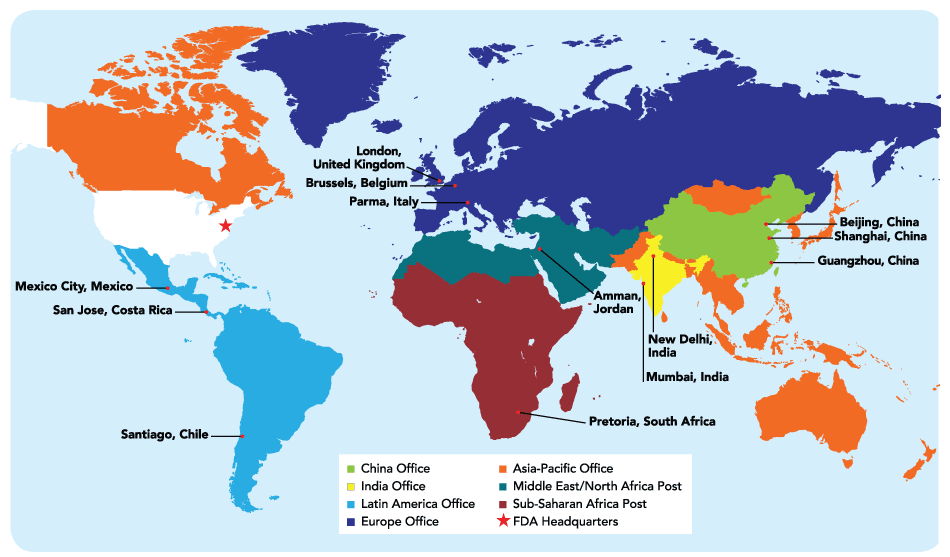
Several FDA foreign offices cover a number of countries, including the post in Amman, Jordan, which is slated to close Dec. 25.
Source: FDA Global Engagement Report
“An Area Of Unique Security And Safety Concerns”
While clearly not of the stature of FDA’s China and India posts, the agency at one point did characterize the Amman office as necessary for supply chain oversight.
It was one of the newest foreign posts, opening in June 2011. The agency said in a February 2012 report![]() to Congress, which was mandated in the Food Safety Modernization Act, that the federal government has “an important interest in Africa through the investment in the President’s Emergency Plan for AIDS Relief,” a program that provides drugs and support to fight the spread of AIDS in the region.
to Congress, which was mandated in the Food Safety Modernization Act, that the federal government has “an important interest in Africa through the investment in the President’s Emergency Plan for AIDS Relief,” a program that provides drugs and support to fight the spread of AIDS in the region.
FDA also said in the report that “the Middle East and North Africa represent an area of unique security and safety concerns and having a person in-region will help FDA better address them.”
FDA wrote in its Global Engagement report![]() , issued in April 2012, that the post covered 20 countries with “varying levels of regulatory maturity” and is “becoming increasingly important” as the number of FDA-regulated products traveling to the U.S. from the region continues to rise. Along with helping local counterparts understand agency regulations, staff in Amman were helping those countries develop systems to assist FDA in finding adulterated and misbranded products shipped from the region, according to the report.
, issued in April 2012, that the post covered 20 countries with “varying levels of regulatory maturity” and is “becoming increasingly important” as the number of FDA-regulated products traveling to the U.S. from the region continues to rise. Along with helping local counterparts understand agency regulations, staff in Amman were helping those countries develop systems to assist FDA in finding adulterated and misbranded products shipped from the region, according to the report.
The agency made clear that it will continue to engage with its partners in the region, but those relationships likely will evolve differently when cultivated from North America rather than locally.
FDA has said it must partner with trusted regulators to share the global supply chain security burden (Also see "FDA Envisions Many Regulatory Coalitions Dealing With Globalization Issues" - Pink Sheet, 26 Sep, 2011.).
Post locations and staffing “are reviewed periodically to determine what changes may be appropriate due to shifts in the global environment, increasing complexities of supply chains, results achieved and budget realities,” FDA noted in the Food Safety Modernization Act report.
The agency continues to think about whether FDA’s foreign presence is correctly positioned “for maximum impact within the global regulatory dynamic,” according to the statement.
Mary Lou Valdez, associate commissioner for international programs, said recently that the agency is asking stakeholders and industry for information on where foreign posts could be located in the future. Among the questions being asked is whether South Africa is the best place for FDA’s office in Africa and whether Thailand is a potential location for a post (Also see "FDA Inspection Growth In China Stymied By Visa Issues" - Pink Sheet, 15 Aug, 2013.).
Further Push Toward System Strengthening
The decision to close the Amman office also may represent further confirmation that FDA no longer wants or expects to inspect all foreign manufacturers around the world. The agency would prefer to help individual countries build their regulatory systems so it can use their information.
“We’re trying to work with all these different regulators, no matter how technically weak or strong they are, just to make sure we don’t have to worry about the imports coming from those countries,” Brykman said.
As part of that effort, FDA is testing a hand-held counterfeit drug detection device in Ghana. Its scientists developed the CD3 to help import security agents quickly scan shipments for potential counterfeit or substandard products (Also see "FDA Counterfeit Screening Tool Gets Field Test In Ghana; Corning Prepares Device For Mass Production" - Pink Sheet, 24 Apr, 2013.).
The agency also now believes that foreign regulator training is not a long-term advantage for its efforts to improve global supply chain security (Also see "Foreign Regulator Training Only Provides Short-Term Gains, FDA Official Says" - Pink Sheet, 9 Sep, 2013.). FDA is focusing on strengthening regulatory systems, rather than training, in its efforts to proactively address supply chain security.
Brykman said the agency also is researching data points that it can use to better predict potential problems and improve regulatory decision-making.
He said the agency has an idea about which data it should collect and is looking at historical problems to see whether it could have reacted earlier. FDA also wants to partner with other regulators that could help with early signal detection.
In addition, the agency is close to creating data-sharing software for regulators to access and share information. Brykman said FDA is “knocking at the door” of developing a web-based platform for real-time information access.
FDA received new authority in the FDA Safety and Innovation Act to increase information sharing with trusted governments, although it is still working to implement the provisions (Also see "FDA Could Release GMP Data To Prod Manufacturing Improvements" - Pink Sheet, 15 Jul, 2013.).
The agency already has a number of agreements in place with the Australian Therapeutic Goods Administration and the European Medicines Agency (Also see "FDA-EMA-TGA Joint Inspection Program To Be Permanent; Other Collaborations Expand" - Pink Sheet, 21 Mar, 2011.).
|
FDA Foreign Offices |
|
|
Country |
Location |
|
Asia |
|
|
China |
Beijing, Shanghai, Guangzhou |
|
India |
New Delhi, Mumbai |
|
Latin America |
|
|
Costa Rica |
San Jose |
|
Chile |
Santiago |
|
Mexico |
Mexico City |
|
Europe |
|
|
Belgium |
Brussels |
|
United Kingdom |
London |
|
Italy |
Parma |
|
Africa and Middle East |
|
|
South Africa |
Pretoria |
|
Jordan |
Amman* |
|
*Scheduled to close Dec. 25. Operations will continue from FDA headquarters. FDA’s Asia-Pacific Office also is located at the Silver Spring, Md., facility. |
|
|
Source: FDA reports on foreign offices |
|
