FDA Office of Regulatory Affairs Make-Over Blends Domestic, International Work
Executive Summary
The reorganized ORA has eight new offices to better handle new legislative authorities and globalization of its regulated products; FDA expects to increase partnerships with other government officials and foreign entities.
FDA is establishing eight new offices within its Office of Regulatory Affairs to better deal with new powers it received from Congress and the globalization of its regulated products.
The new offices are part of a reorganization of ORA that became effective Oct. 1. The offices largely consolidate responsibilities previously carried out in other offices and divisions and increase their visibility and profile.
“FDA faces a time of remarkable transformation due to the rapid modernization and globalization of our regulated products, and the new legislative authorities provided by Congress,” the agency states in a fact sheet on the organization. “ORA’s headquarters reorganization is a positive step forward to better position us to meet those new demands and better protect public health.”
FDA explained that the make-over will enable ORA to operate more effectively, gather better data for analysis, create more strategic coalitions and increase partnerships with federal, state and local officials and foreign entities.
Specifically, the ORA reorganization is intended to help the agency carry out mandates provided in the Food Safety Modernization Act and the FDA Safety and Innovation Act, which reauthorizes user fees for prescription drugs and includes provisions to enhance benefit-risk assessment and regulatory science.
“The new offices remove the current domestic/international distinctions,” Steven Lynn, director of the Office of Manufacturing and Product Quality in the Center for Drug Evaluation and Research’s Office of Compliance, said Sept. 25 at Xavier University’s global outsourcing conference. “They’ve actually been combined to a big part. And border enforcement and compliance systems have been combined, reflecting our move to a global approach to our operations.”
Below are charts of ORA before and after the reorganization and a box of those appointed to head up the new offices. The first chart includes recent appointments not noted in the organization chart posted on the agency’s website. FDA has also just completed a more detailed reorganization chart.
REGULATORY REORG: The Revised Look Of The Office Of Regulatory Affairs
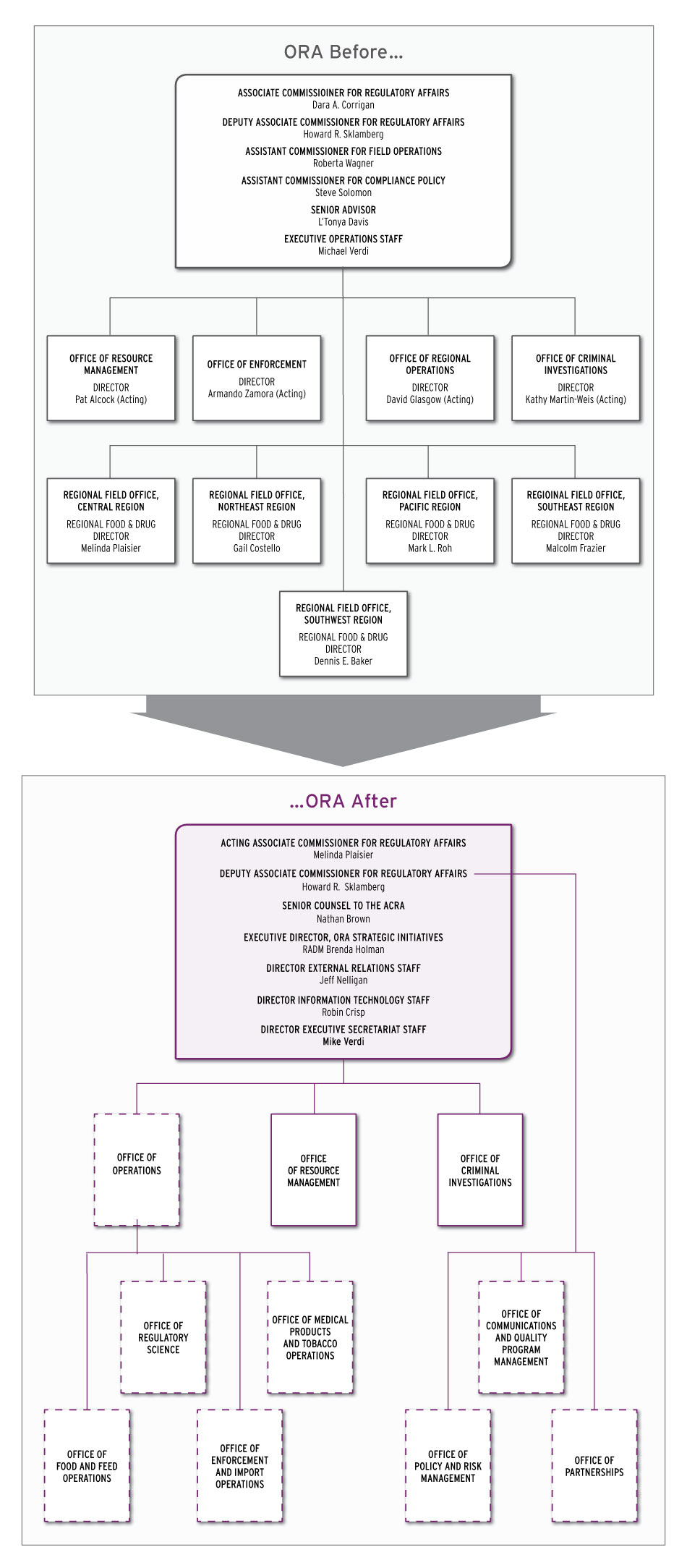
ORA's new offices are indicated with a dotted line border.
ORA’s New Office Leaders
Office of Operations: Assistant Commissioner for Operations Ellen Morrison
Office of Enforcement and Import Operations: Acting Director Ricky Rodriguez
Office of Food and Feed Operations: Acting Director Charles Breen
Office of Medical Products and Tobacco Operations: Acting Director Paul Teitell
Office of Regulatory Science: Acting Director Mark Madson
Office of Communications and Quality Program Management: Director L’Tonya Davis
Office of Policy and Risk Management: Acting Assistant Commissioner for Policy Leigh Verbois
Office of Partnerships: Acting Director Laurie Farmer
The previous Office of Enforcement and Office of Regional Operations will be replaced with three new offices:
- Office of Food and Feed Operations;
- Office of Medical Products and Tobacco Operations; and
- Office of Enforcement and Import Operations, which combines most of the functions of the Office of Enforcement and the operations of ORO’s Division of Import Operations and Policy.
The Office of Food and Feed Operations and the Office of Medical Products and Tobacco Operations assume most of the functions of the Office of Regional Operations’ Division of Domestic Field Operations and the Division of Foreign Field Operations.
The other new offices are:
- Office of Regulatory Science, which will carry forward most of the functions of the old Division of Field Science and include staff that specialize in tobacco, medical products or food and feed;
- Office of Operations, which will provide support for inspections, compliance, laboratory and field operations. Four of the new offices will report directly to the Office of Operations as will ORA’s five regional offices and its laboratories.
- Office of Policy and Risk Management, which will consolidate policy staff from various ORA HQ components and report to the Deputy ACRA;
- Office of Communications and Quality Program Management, which will consist of internal communications, project coordination and quality management staff and report to the Deputy ACRA; and
- Office of Partnerships, which includes the division of federal-state relationships, and which also will report to the Deputy ACRA.
The existing Office of Resource Management will remain in place and include a new Division of Budget Formulation and Execution.
The office of the Associate Commissioner for Regulatory Affairs itself has been undergoing a transformation. FDA previously announced that Dara Corrigan was leaving as Associate Commissioner to become director of FDA’s Europe Office and Senior Advisor for Global Operations, based in Brussels. Melinda Plaisier was appointed to the associate commissioner post on an acting basis (Also see "FDA Associate Commissioners Transition As PDUFA IV Rolls Over To V" - Pink Sheet, 13 Sep, 2012.).
The ORA revamp is among other recent changes within the agency focused on product quality. Last month, the agency announced plans to move its 11-member Drug Shortages Staff from the Office of New Drugs into the Office of the Center Director (Also see "FDA Drug Shortage Staff Gets Higher Profile As New Reporting Requirements Begin" - Pink Sheet, 28 Sep, 2012.). In addition, it elevated the Office of Generic Drugs to “super office” status (Also see "Generic Drugs Gain Status Boost Within CDER" - Pink Sheet, 7 Sep, 2012.).
The changes in ORA structure appear to be a reconfiguration of existing staff and resources. FDA said the reorganization will not result in any job losses or physical moves within the agency. This restructuring is far different from the previous overhaul of the office proposed in 2007. At that time, the agency planned to shut down all its regional offices and close several field labs, but cancelled the plans amid opposition from Congress (Also see "FDA Takes “Fresh Look” At ORA Reorg, Eyes Outsourcing Some Agency Posts" - Pink Sheet, 27 Aug, 2007.).
FDA noted that the current reorganization will not make any structural changes to the regional or district offices, laboratories, or the Office of Criminal Investigations.
