International Group Finds Harmony In Benefit-Risk Assessment Framework
Executive Summary
Sponsors and regulators continue to develop methodologies with a range of complexity for presenting, communicating and discussing a drug’s risks and benefits.
An international group of regulators and drug companies have agreed in principle to a framework that sets out eight steps for assessing a drug’s benefits and risks and could set the stage for a global approach to evaluating drugs.
The framework will not result in uniform decisions across countries, but rather will provide a structure for the benefit-risk assessment process as a number of efforts are underway to make the exercise more methodical and to develop systematic ways for regulators and sponsors to present, communicate and discuss drug data.
Methodologies to reach decisions may vary, but “if everybody follows those same basic eight steps, … any new method that you come up with to develop or to assess benefit-risk should be internationally acceptable because they follow these general eight-step principles,” Lawrence Liberti, executive director of the Centre for Innovation in Regulatory Science, explained in an interview.
UMBRA Eight-Step Benefit-Risk Assessment Framework
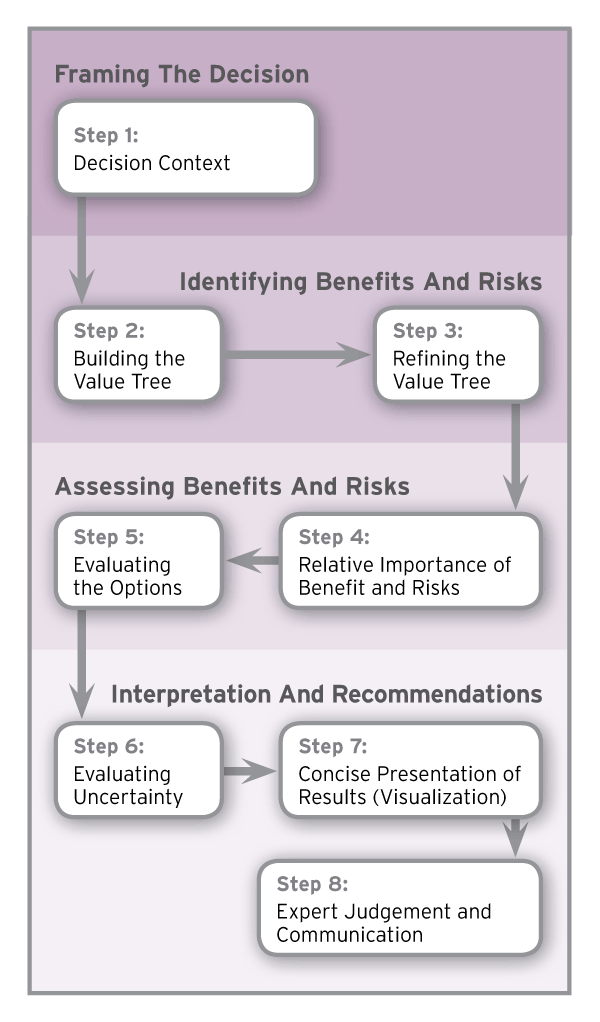
Source: Centre for Innovation in Regulatory Science
A task force of representatives from eight regulatory agencies and six international pharmaceutical companies, organized by CIRS through its Unified Methodologies for Benefit-Risk Assessment initiative, endorsed the framework following a June 21-22 workshop held in Washington, D.C., to discuss global harmonization of the benefit-risk assessment process. FDA Senior Advisor Murray Lumpkin and GlaxoSmithKline Inc. Senior VP-Global Clinical Safety and Pharmacovigilance Frank Rockhold co-chaired the workshop of regulators, academics and industry representatives.
The CIRS-mediated effort is separate from the International Conference on Harmonization, a long-standing initiative to coordinate regulatory standards between the U.S., Europe and Japan. FDA recently put ICH periodic benefit-risk evaluation standards into draft guidance for post-market safety reporting (Also see "Harmonization Fail: ICH Guideline Doesn’t Simplify Safety Reporting, Firms Say" - Pink Sheet, 30 Jul, 2012.).
While the eight steps coming out of the CIRS effort create structure, the methodology for benefit-risk decision-making will not be uniform soon, if ever. A common lexicon should be developed, according to a synopsis of the meeting, which was not open to the public. But agencies vary in their weighting of benefit-risk parameters and there are regional differences in regulatory and cultural viewpoints, making uniformity difficult.
Liberti also pointed out that different tools may be needed for drugs in different therapeutic categories, or at different times in a drug’s life cycle, such as development versus post-market. “There’s no single method that’s best used for every kind of benefit-risk decision.” But while the tool may look different, “we can always map it back to that standard framework of decision making,” he said.
Participants In Benefit-Risk Task Force
- Food and Drug Administration
- European Medicines Agency
- Swissmedic
- Health Canada
- Danish Medicines Agency
- Health Sciences Authority, Singapore
- Medicines and Healthcare products Regulatory Agency, U.K.
- Therapeutic Goods Administration, Australia
- AstraZeneca
- Pfizer
- Johnson & Johnson
- Pharmaceutical Research And Manufacturers of America
- Eli Lilly & Co.
- GlaxoSmithKline
- Bristol-Myers Squibb
- European Federation of Neurological Associations
With general agreement on the framework, stakeholders now can focus their attention on developing those methodologies. “Time should be allowed for pragmatic methodological approaches to be developed, including adequate timing for feedback on best practices to emerge,” the synopsis says.
Evolving Methodologies
Several methodologies have been, or are being, tested. CIRS’ UMBRA project produced a methodology called “proforma” that the organization is now integrating with one developed by Pharmaceutical Research and Manufacturers of America’s Benefit-Risk Assessment Team (Also see "Benefit-Risk Assessment Framework Moves Toward Global Harmonization" - Pink Sheet, 30 Jan, 2012.).
Four agencies that make up the Consortium on Benefit-Risk Assessment – Swissmedic, Health Canada, Singapore’s Health Sciences Authority and Australia’s Therapeutic Goods Administration – pilot-tested the methodology using information from applications for a drug they previously approved.
The results are not ready for release, but Liberti said the test “was very very positive in the sense that the four agencies found the proforma approach gave them a clear way to identify the most critical benefits and risks and to share those with each other in a very simple manner.”
The BRAT process was pilot-tested by 13 companies during various stages of drug development. A case study of its application to evaluate Johnson & Johnson/Bayer HealthCare AG’s rivaroxaban found that such methodology can add rigor and transparency to decision-making and is easily used in regulatory settings, such as advisory committee meetings, according to the workshop synopsis.
Now, CIRS is taking the best elements of the BRAT methodology and combining them with the best elements of proforma. While proforma is descriptive and verbal, the BRAT methodology provides greater visualization (Also see "PhRMA Testing Pilot Framework For Structured Benefit-Risk Assessment" - Pink Sheet, 12 Sep, 2011.).
The result will be the Benefit-Risk Assessment Support System. CIRS expects the first iteration of BRASS to be available for pilot testing Jan. 1.
FDA GRID In Field Test
FDA is field testing its benefit-risk framework with six products. With the FDA approach, reviewers list evidence/uncertainties and conclusions/reasons for five decision factors in a grid format and then analyze the implications. The factors are severity of condition, unmet medical need, clinical benefit, risk and risk management.
The agency aims to evaluate and further refine the system so it can be implemented into the drug review process, Center for Drug Evaluation and Research Office of Planning and Analysis Director Patrick Frey told the workshop.
FDA committed to a structured benefit-risk assessment framework for the drug review process as part of the user fee agreement incorporated into the FDA Safety and Innovation Act (Also see "FDASIA Is Signed, Not That White House Wanted Anyone To Notice" - Pink Sheet, 16 Jul, 2012.).
EMA’s Qualitative Tool
The European Medicines Agency has a benefit-risk assessment methodology that it considers a simple qualitative tool. PrOACT-URL identifies the problem, determines the objective, considers the alternatives and their consequences (presented in tabular form) and makes tradeoffs through swing-weighting of the events. Sensitivity analysis determines the level of uncertainty.
In a field test using AstraZeneca UK Ltd.’s Caprelsa (vandetanib) and placebo as the alternatives for treating medullary thyroid cancer, the risk tolerance found was reflected by restrictive approval for use in a limited set of patients, reported Francesco Pignatti, head of Oncology Safety and Efficacy of Medicines at EMA. The agency hopes to institute a pilot program to determine if the effects table is generally fit for purpose, he said.
While tools such as PrOACT-URL should be implemented, EMA senior medical officer Hans-Georg Eichler told the workshop, stakeholders should explore more complex, quantitative tools and determine how to combine patient opinions and the diverse spectrum of value judgments with the technical expertise of regulatory scientists.
Patient Benefit-Risk Input
Both regulators and drug developers are intent on gathering more input from patients on what benefits are important and how much risk can be tolerated, as evidenced by the FDA/industry user fee agreement to obtain patient perspectives during drug development (Also see "Getting a Jump on PDUFA V: Fast Start on REMS, Patient Input – And Longer Reviews" - Pink Sheet, 1 Jun, 2012.).
Among FDA initiatives in this area is a basic roadmap to be used by patient groups interested in development of patient-reported outcome measures in a specific disease area, Director of CDER’s Office of Planning and Informatics Theresa Mullin reported.
A scenario presented to the workshop calls for patients, during the translational period between drug discovery and clinical studies, to identify important dimensions of benefit not captured in current studies and the need for PRO instruments, which would be developed and qualified during the translational period through Phase I. During the clinical development period, patients would give their opinion on the effectiveness and tolerability of currently available therapies; patient input would continue during the post-market period.
On the developer’s side of the table, Diana Hughes, a vice president in Pfizer Inc.’s primary care business unit, suggested industry form a consortium with the mission of gaining a perspective on unmet medical need and patient experience. Companies should continue collaborating with advocacy groups and develop patient educational programs to elicit information on the most relevant aspects of a disease and advance a common approach to valuing and weighting benefit and risk, she said.
Workshop participants concluded that rules of engagement must be set to avoid any misperceptions of conflict-of-interest during interactions with patients, and that such interactions are consistent, scheduled and balanced. Patients would benefit from education on the inherent nature of uncertainty in benefit-risk decisions, according to the workshop.
CIRS is part of the Intellectual Property and Science business of Thomson Reuters, but is operated as an independent company for the sole support of its members’ activities; funding includes dues from 22 pharmaceutical companies.