CBER New Biologic Tally Healthy In 2013, But For Innovation Look To 2014
This article was originally published in Pharmaceutical Approvals Monthly
Executive Summary
Coagulation factors dominated the biologics center’s approvals in 2013, a solid year with eight novel BLAs cleared. Innovation level of 2013 CBER approvals was unremarkable, but first quarter of 2014 holds potential for advances in hemophilia and allergy treatment.
FDA’s Center for Drug Evaluation and Research may have approved an anemic total of two new BLAs in 2013, but the Center for Biologics Evaluation and Research posted one of its best years for novel approvals since FDA transferred therapeutic biologics to the drugs center a decade ago, clearing eight novel products.
CBER’s approvals, however, offer an unremarkable level of innovation – only two of the eight received priority review -- while both CDER therapeutic biologics received priority review; Genentech Inc.’s Gazyva (obinutuzumab) is one of the first “breakthrough” therapies to clear the agency and the company’s antibody-drug conjugate Kadcyla (ado-trastuzumab) is first in its class (see related story, (Also see "New Drugs In 2013 Show A Virtuous Cycle: First-Cycle Approvals Up, Review Times Down" - Pink Sheet, 21 Jan, 2014.)).
Since review responsibility for therapeutic biologics moved from CBER to CDER in late 2003, the novel BLA tally for the products remaining in the biologics center, including blood and plasma products, vaccines, and antitoxins, has fluctuated from a low of two to a high of nine (see graph). The eight 2013 approvals were exceeded only in 2009, and represent a recovery from a low in 2011 and modest 2012.
Biologics From The Biologics Center
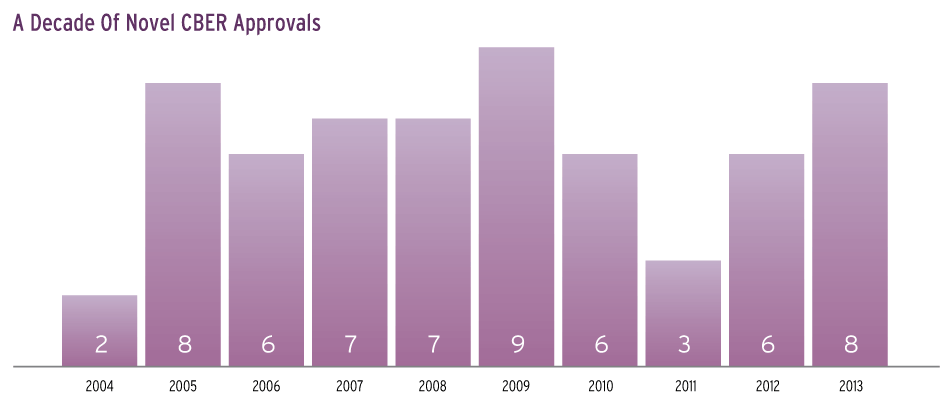
Novel BLAs approved by CBER after the transfer of therapeutic biologics to CDER
CBER, like CDER, met all of its user fee goals for its class of 2013 novel agents. But reviews at the biologics center took longer than at the drugs center – an average of 20.75 months from submission to approval at CBER, compared with 13.2 months at CDER, or a median of 13 months vs. 10 months.
Only one CBER approval was submitted under the PDUFA V review “program,” which extends the user fee goal for novel submissions after Oct. 1, 2012 and increases communication. Novo Nordisk AS’ NovoEight (recombinant antihemophilic factor) moved through the agency efficiently but did not present a significant review challenge – it belongs to a class of recombinant Factor VIII products marketed since 1992.
Priority Products Have Limited Commercial Appeal
The two priority-reviewed BLAs that cleared CBER in 2013 are not likely to make a huge impact on the sales front, although they occupy important public health niches.
Protein Sciences Corp.’s recombinant Flublok is the first influenza vaccine produced without the use of eggs or live influenza virus, allowing for much faster manufacturing – a valuable attribute in the event of a pandemic, and one that drew the Biomedical Advanced Research and Development Authority into a partnership with Protein Sciences (Also see "Protein Sciences To Begin Selling Novel Flu Vaccine; Flublok Has Virus-Free Appeal" - Pink Sheet, 17 Jan, 2013.). But in commercial terms, Flublok started out with some drawbacks: it is only approved for people 18 to 49 years old; it is a trivalent formulation in a market moving toward quadrivalent vaccines; and it has a shelf-life of 16 weeks compared with the typical 12-month shelf-life of most inactivated trivalent flu vaccines.
BARDA also supported the development of Cangene Corp.’s heptavalent botulism antitoxin for treatment of symptomatic botulism. BAT qualified for priority review because it filled an unmet medical need – no treatment for adult botulism has been available since the last lot of Sanofi Pasteur’s bivalent botulism antitoxin expired in early 2010. Cangene’s BAT was CBER’s first product approved under FDA’s “animal rule” allowing approval based on animal data when human efficacy studies are not ethical or feasible.
BAT, like another BARDA-supported 2013 approval, GlaxoSmithKline PLC’s adjuvanted H5N1 influenza vaccine, is part of the U.S. National Stockpile. The GSK H5N1 vaccine, also known as Q-Pan H5N1, is the second “bird flu” vaccine approved for the stockpile as part of national pandemic preparedness initiatives (Also see "New Biologics Total Seven In 2007, But Only Four Will See Market" - Pink Sheet, 1 Jan, 2008.). The H5N1 vaccines are not promoted or distributed commercially.
GSK’s H5N1 vaccine was one of the longer CBER reviews of 2013, at 21 months and two review cycles. The March 22, 2013 “complete response” letter reflected GSK’s “inadvertent” failure to submit the full data package for the pivotal study; 42 datasets and 71 case report forms were not submitted, FDA review documents report. Technical problems with the applications also prolonged review of two GSK NMEs approved in 2013 (Also see "Technical Difficulties Prevented Priority Review Of GSK Melanoma Drugs" - Pink Sheet, 15 Oct, 2013.).
A Conglomeration Of Coagulation Factors
The 2013 CBER approvals were dominated by coagulation factors, all receiving standard review, including new recombinant options for Factor VII, IX and XIII replacement as well as two plasma-derived products providing replacement of multiple coagulation factors (see chart).
CSL Behring’s Kcentra (prothrombin complex concentrate) is the only first-in-class coagulation product cleared by CBER in 2013. The four-factor concentrate was cleared for urgent reversal of vitamin K antagonist-induced acquired coagulation factor deficiency in patients with acute major bleeding in April under standard review, and then expanded use to patients about to undergo surgery or invasive procedures in a priority review sBLA approved Dec. 13, 2013.
While Kcentra is the first nano-filtered PCC for urgent warfarin reversal in the U.S., where only plasma had been approved, the product has been marketed overseas since 1996. The other 2013 CBER approval for replacement of multiple coagulation factors, Octapharma AG’s Octaplas (pooled human plasma, solvent-detergent treated), is also no stranger to the world market: as OctaplasLG, it has been available since 2009.
Octapharma is looking to bring an established PCC to the U.S. for warfarin reversal as well; it has marketed Octaplex overseas since 2003. It is also hoping to bring the big marketing guns: Pfizer Inc. will market the PCC, a potential competitor to Kcentra, under a deal announced Dec. 11 [See Deal]. Octapharma submitted a BLA in April 2013 for an urgent surgery claim, putting the likely goal for FDA action in the spring of 2014.
Approval Activity Across Hemophilia Markets
The bleeding disorder market has moved steadily from plasma-derived factors to recombinant versions after concerns about blood-born virus transmission in the wake of the HIV and hepatitis epidemics of the 1980s.
Novo Nordisk’s Tretten (coagulation Factor XIII A-Subunit, recombinant) is the first recombinant agent for routine prophylaxis in patients with congenital Factor XIII A-subunit deficiency routine prophylaxis. FDA considered it a standard review product for the ultra-orphan market.
Novo’s other 2013 approval, NovoEight, targets the largest of the orphan hemophilia markets, hemophilia A, or patients who lack clotting Factor VIII. Recombinant FVIII has been available since the mid-1990s; NovoEight joins a third generation of rFVIIIs, dominated by Baxter Healthcare Corp.’s blockbuster Advate, that do not contain any human or animal plasma proteins in the final formulation.
Baxter’s Rixubis is the first new recombinant Factor IX in 15 years for the even rarer disorder hemophilia B, and the first rFIX approved for both routine prophylaxis and control of bleeding episodes. FDA issued a “complete response” letter for Cangene Corp.’s rFIX IB1001 in February 2013.
Baxter also received a new indication for Feiba (anti-inhibitor coagulant complex) on Dec. 19 for routine prophylaxis of bleeding episodes in patients with hemophilia A or B who have developed inhibitors that reduce efficacy of standard treatment. The inhibitor or bypass market is the fastest-growing segment of the hemophilia market .
The Grastek and Oralair advisory committee review Dec. 11 and 12 recommended the oral products be co-packaged with epinephrine, which could be a hurdle for the sponsors’ message of safety and convenience of the new products compared with traditional allergenic extracts injected by physicians (Also see "Merck, Stallergenes Oral Allergy Immunotherapies Likely To Require Epi" - Pink Sheet, 13 Jan, 2014.). Merck’s ragweed allergy immunotherapy Ragwitek will be reviewed by the committee Jan. 28.
Even co-packaged with epi, oral allergy immunotherapy offers the same sort of potential for a major advance in dosing and convenience, with the potential to bring new patients to routine product use, that the long-acting coagulation factors aim to offer hemophilia patients. On both fronts, CBER looks likely to see a 2014 that meets or exceeds its record of new product approvals in 2013.
