Drug Product Recalls in 2013 Categorized by Problem Area
This article was originally published in The Gold Sheet
Executive Summary
This comprehensive table provides key facts at a glance for every one of the 1,276 drug recalls FDA reported last year, grouped into seven main problem areas. For each recall, it says what product was recalled and how much of it was recalled, what company manufactured it and recalled it, how seriously FDA rated the health threat the recalled product posed, and what reason the company gave for recalling the product.
The information on drug product recalls in the table below was collected and categorized by “The Gold Sheet” from weekly FDA “Enforcement Reports” issued during calendar year 2013.
FDA indicates in the reports whether it regulates recalled products as drugs, biologics, medical devices/diagnostics, foods, or veterinary products. The list below includes only those recalls involving drug products and products making drug claims. Food supplements and biologic products, for example, have not been included.
The recalls are grouped into seven categories based on the reasons for them, starting with the most common reason and ending with the least common: Contamination; manufacturing/testing methods; labeling/packaging mix-ups; other product specifications; potency/content uniformity; compliance with NDA/monograph requirements; and dissolution.
In each category, recalls are listed by class, and in each class, by the date the recall began, which can precede FDA’s recall report by weeks or months.
FDA designates a recall as falling into one of three classes according to the health threat posed by the product problem: Class I – violative product poses reasonable probability of serious adverse health consequences or death; Class II – violative product may cause temporary or medically reversible adverse health consequences, probability of serious consequences remote; Class III – violative product not likely to cause adverse health consequences.
For analysis of the recalls tabulated here, see the article in this issue (Also see "Drug Recalls Soared Again in 2013, Driven by Contamination" - Pink Sheet, 30 May, 2014.).
“The Gold Sheet” has also compiled an Excel file with additional information that can be filtered by report date, recall date, dosage form, the reason for the recall and whether the product was available by prescription or over the counter.
Follow this link to use the Excel spreadsheet of 2013 drug recalls.
Find answers in recalls spreadsheet
Click here to open the 2013 drug recalls spreadsheet.
|
Contamination |
|||
|
Product(s)
|
Recaller / Manufacturer |
Class
|
Reason
|
|
Methylprednisolone Acetate (PF) 80 mg/ml Injection, supplied in 23,897 1 mL, 2 mL and 5 mL vials; Methylprednisolone Acetate (PF) 40 mg/ml Injection, supplied in 9,062 1 mL, 2 mL and 5 mL vials; Methylprednisolone Acetate 40 mg/mL Injection, Preserved, supplied in 5,130 5 mL and 10 mL vials; Methylprednisolone Acetate 80 mg/mL Injection, Preserved, supplied in 3,822 5 mL and 10 mL vials |
New England Compounding Center, Framingham, MA |
I |
Non-Sterility |
|
Trastuzumab Kit containing 1 20 mL multidose vial Trastuzumab 440 mg, investigational use only, 2,140 kits |
Genentech Inc., South San Francisco, CA / Hospira Inc. |
I |
Presence of Particulate Matter: One lot of Bacteriostatic Water for Injection, USP diluent vials that were packed with Trastuzumab Kits for investigational use has the potential to contain glass particulates. |
|
Isovue 370 (lopamidol) Injection 76%, Prefilled 10 x75 mL power injector syringe, 5,483 syringes; Isovue 300 (lopamidol) Injection 61% Prefilled 10 x 100 mL Power Injector Syringe, 5,736 syringes |
Bracco Diagnostic Inc., Princeton, NJ / Nycomed Gmbh, Singen, Germany |
I |
Presence of Particulate Matter; fibers identified as cellulose and polyvinyl |
|
Zicam Extreme Congestion Relief gel spray, oxymetazoline HCl nasal gel, 0.05%, 15 mL spray bottle, 46,752 bottles |
Matrixx Initiatives Inc., Scottsdale, AZ |
I |
Microbial Contamination of Non-Sterile Products: Product may be contaminated with Burkholderia cepacia. |
|
136,224 Lactated Ringer's and 5% Dextrose Injection, USP, 1000 mL flexible containers |
Hospira Inc., Lake Forest, IL |
I |
Non-Sterility: One confirmed customer report that product containted spore-like particulates, consistent with mold. |
|
143,136 Lactated Ringer's and 5% Dextrose Injection, USP, 1000 mL flexible containers |
Hospira Inc., Lake Forest, IL |
I |
Non-Sterility: Confirmed customer complaint of product contaminated with mold. |
|
Methylprednisolone Preservative Free 40 mg/ml Injectible Suspension, 1,395 vials; Cyanocobalamin 1000 mcg/ml, 58 30-mL multi-dose vials |
Green Valley Drugs, Henderson, NV |
I |
Non-Sterility: Green Valley Drugs received positive sterility results from their testing lab on two lots of Methylprednisolone Preservative Free 40mg/mL injectable suspension and one lot of Cyanocobalamin 1000 mcg/mL injection, 30mL MDV. |
|
Vistide (cidofovir) Injection, 375 mg, 3,181 5mL single-use vials |
Gilead Sciences, Inc., Foster City, CA |
I |
Presence of Particulate Matter: Particulate matter was found in some vials of Vistide. |
|
Mitosol 0.2mg/vial, Kit for Ophthalmic use, 3 kits/box, 83 boxes |
Mobius Therapeutics LLC., Saint Louis, MO |
I |
Non-Sterility: one or more components of the kit have been found to be contaminated with yeast. |
|
Magnesium Sulfate 2 grams in Dextrose 5% for Injection, in 403,883 50mL Plastic Infusion Bags |
Med Prep Consulting, Tinton Falls, NJ |
I |
Non-Sterility; mold contamination. |
|
0.9% Sodium Chloride Injection, USP, 691,356 1000mL flexible containers |
Hospira Inc., Lake Forest, IL |
I |
Presence of Particulate Matter: reports of small grey/brown particles found in the primary container identified as brass particulates. |
|
Methotrexate Injection, USP, Preservative-Free, 1g/40mL, 1,635 40mL Single Dose Vials |
Sandoz Inc., Broomfield, CO / EBEWE Pharma, Unterach, Austria |
I |
Presence of Particulate Matter: Found during examination of retention samples. |
|
0.9% Sodium Chloride Injection, USP, 264,432 100ml bags |
Hospira Inc., Lake Forest, IL |
I |
Presence of Particulate Matter; product may contain fibrous material. |
|
Magnesium Sulfate Injection, USP, 50% for IM or IV use, 15,625 preservative-free single-dose vials |
Fresenius Kabi USA, Lake Zurich, IL / APP Pharmaceuticals Schaumburg, IL |
I |
Presence of Particulate Matter: Glass particulate matter was observed in a retention sample during an annual review. |
|
Carboxymethylcellulose Sodium 0.5% Ophthalmic Solution, various store brands, 363,746 1fl oz bottles |
Altaire Pharmaceuticals, Inc., Aquebogue, NY |
I |
Non-Sterility: Customer complaints of mold in the product after use and handling due to the fact that the preservative used in the lots of Carboxymethylcellulose Sodium 0.5% Ophthalmic Solution may not be effective through expiry. |
|
0.25% Bupivacaine HCI Injection, USP, 2.5mg/ml, 30mL, 118,100 single-dose vials; and 0.75% Bupivacaine HCI Injection, USP, 7.5mg/ml, 30mL, 119,600 single-dose vials |
Hospira Inc., Lake Forest, IL |
I |
Presence of particulate matter: visible free floating and partially embedded particulate matter in the glass vials. |
|
Cymevene 500 mg Powder for Infusion, (ganciclovir), 50 mg/mL ganciclovir (reconstructed), 42,010 10mL vials |
F. Hoffmann-LaRoche Ltd., Basel, Switzerland / Roche Products Limited, Welwyn Garden City, AL7 1TW, United Kingdom |
II |
Lack of Assurance of Sterility; container closure issues with the bulk batch. |
|
13 injectables recalls *
|
New England Compounding Center, Framingham, MA |
II |
Lack of Assurance of Sterility |
|
* Betamethasone Repository 6 mg/mL PF Injection and Betamethasone Repository 6 mg/mL P Injection; Betamethasone Sodium Phosphate 6 mg/mL P Injection, Betamethasone Sodium Phosphate 6 mg/mL PF Injection, Betamethasone Sodium Phosphate 8 mg/mL PF Injection, Betamethasone Sodium Phosphate 12 mg/mL PF Injection; Bupivacaine 0.25% PF, Bupivacaine 0.5% PF, and Bupivacaine 0.75% PF; Clonidine 100 mcg/mL PF, 2,747 1mL and 2 mL vials; Dexamethasone Sodium Phosphate, 20 vials, 4 mg/mL vials PF, 6 mg/mL PF and 8 mg/mL PF; Glycerin 100% PF, 165 vials, 1mL, 2 mL, and 5 mL; Hyaluronidase, 10,865 vials, 150 u/ml PF, 1 mL, 5 mL, and 10 mL; Isovue 200 mg/mL PF and 300 mg/mL PF, 7,055 vials; Lidocaine/Dextrose 5%/7.5% PF; Lidocaine 1% PF, 2% PF, and 4% PF, in 3,321 1 mL, 2 mL, 5 mL, and 10 mL vials; Omnipaque 240mg/mL PF, 300mg/mL PF, in 8,919 3 mL, 5 mL, and 10 mL vials; Saline 10% and 3%, in 54 5 mL and 10 mL vials; Triamcinolone 10 mg/mL P, 40mg/mL P, 40mg/mL PF and 80 mg/mL PF in 56,655 1 mL, 2 mL, 5 mL, and 10 mL vials. |
|||
|
Epinephrine Injection USP, 1 mL, USP, 361,600 1mL ampules |
Hospira, Inc., Lake Forest, IL |
II |
Presence of Particulates; may contain glass particles |
|
I-C Drops, Eye Cleansing Agent, 15mL, 253 bottles |
James G.Cole, Inc., Hood River, OR / ASN/Maxam P.O. Box 1277 Hood River, OR |
II |
Lack of Assurance of Sterility: The product is being recalled because the product label does not state sterile in accordance with 21 CFR Part 200.50, Ophthalmic Preparations and Dispensers, and it is not in compliance with 21 CFR Part 349, Ophthalmic Drug Products for Over-The-Counter Use. |
|
Prednisone tablets, USP, 10mg, 86,616 tablets |
L. Perrigo Co., Allegan, MI / West-ward Pharmaceutical Corp., Eatontown, NJ |
II |
Presence of Foreign Substance(s); Perrigo has been notified of a recall by the manufacturer of this product, West-Ward Pharmaceuticals. This is a sub-recall due to tablets contaminated with trace amounts of food-grade lubricant, as well as stainless steel inclusions |
|
Lisinopril Tablets, USP 40mg in 100-count and 1000-count bottles, 32,550 bottles |
West-ward Pharmaceutical Corp., Eatontown, NJ, and Blu Pharmaceuticals, Franklin, KY / West-ward Pharmaceutical Corp., Eatontown, NJ |
II |
Presence of Foreign Substance: Uncharacteristic black spots identified as a food grade lurbicant with trace amounts of foreign particulates and stainless steel inclusions have been found in the tablets. |
|
Propylthiouracil Tablets, USP, 50mg in 100 count and 1000 count bottles, 43,075 bottles; Lisinopril and Hydrochlorothiazide Tablets, 20mg/25mg in 100 count and 1000 count bottles, 43,478 bottles; and PredniSone Tablets, USP, 20mg 100 count tablets, 500 count tablets, 1000 count tablets per bottle, 60,289 bottles |
West-ward Pharmaceutical Corp., Eatontown, NJ |
II |
Presence of Foreign Substance: Uncharacteristic spots identified as steel corrosion, degraded tablet material and hydrocarbon oil with trace amounts of iron were found in tablets |
|
Hillyard, Alcohol Free Foaming Instant Hand Sanitizer Chloride 0.10% plastic bottle, 2,139 bottles |
Hillyard GMP, Saint Joseph, MO / Hillyard Industries, St. Joseph, MO |
II |
Microbial Contamination of Non-Sterile Products: The product may be contaminated with bacteria. |
|
PredniSone Tablets, USP, 10mg, 100 and 1,000 per bottle, 128,319 bottles |
West-ward Pharmaceutical Corp., Eatontown, NJ |
II |
Presence of Foreign Substance: Tablets are being recalled due to gray defects identified in the tablets. |
|
Carisoprodol Tablets, USP, 350 mg, 500 to 1,000 per bottle, 8,585 bottles |
West-ward Pharmaceutical Corp., Eatontown, NJ |
II |
Presence of Foreign Substance: Uncharacteristic black spots on tablets |
|
Lansoprazole Delayed-release capsules, USP, 30 mg, 500-count capsules, 1,894 bottles |
Mylan Pharmaceuticals Inc., Morgantown, WV / Made in India for Mylan Pharmaceuticals Inc., Morgantown, WV |
II |
Presence of Foreign Tablets/Capsules: Bottles of Iansoprazole 30mg delayed-release capsules may contain topiramate 100 mg tablets. |
|
Propofol Injectable Emulsion 1%, single patient infusion vials, various dosages, 273,925 vials |
Hospira, Inc., Lake Forest, IL |
II |
Presence of Particulate Matter: Visible particulate and particulate embedded in vials were observed during retain inspection. |
|
12-hour Sinus (oxymetazoline hydrochloride) Nasal Spray, 0.05%. 1fl oz, 32,460 bottles |
Lee Pharmaceuticals Inc., South El Monte, CA |
II |
Microbial Contamination of a Non-Sterile Products: 12-hour sinus nasal spray under various labeling are being recalled due to microbial contamination identified during testing. |
|
Citalopram tablets USP, 10, 20 and 40 mg, 30-count bottles: 27,805, 86,093 and 96,274 bottles, respectively |
Dr. Reddy's Laboratories, Inc., Bridgewater, NJ / Dr Reddy's Laboratories Limited, Bachupalli, India |
II |
Chemical Contamination: The product is being recalled due to complaints reporting a strong garlic odor or strong chemical smell. |
|
Furosemide Injection USP, 10 mg/mL, 4 mL in 63,900 5mL fliptop vials |
Hospira, Inc., Lake Forest, IL / Hospira, Inc., Rocky Mount, NC |
II |
Lack of Assurance of Sterility; possible loose crimp applied to fliptop vial. |
|
Belladonna Alkaloids (hyoscamine sulfate, atropine sulfate and scopolamine HBr) with phenobarbital tablets 1000 count and 5000 count bottles, 6,511 bottles in all |
West-ward Pharmaceutical Corp., Eatontown, NJ |
II |
Presence of Foreign Substance: Uncharacteristic blacks spots were found in tablets. |
|
0.9% Sodium Chloride Irrigation, USP, 3000 mL, 56,854 units |
Hospira, Inc., Lake Forest, IL |
II |
Lack of Assurance of Sterility: There is a potential for the solution to leak from the administration port of the primary container. |
|
Hextend 6% Hetastarch in Lactated Electrolyte Injection, 500 mL, 11,412 bags |
Hospira,Inc., Lake Forest, IL |
II |
Lack of Assurance of Sterility: There is a potential for the solution to leak from the seal of the fill tube to the bag. |
|
0.9% Sodium Chloride Injection USP, 100 mL, 325,056 bags |
Hospira, Inc., Lake Forest, IL |
II |
Lack of Assurance of Sterility: There is the potential for solution to leak from the administrative port to the fill tube seal. |
|
Terazosin HCl Capsules, 2 mg, in 100-count and 1,000-count bottles, 3,913 bottles |
Teva Pharmaceuticals USA, Sellersville, PA / Intas Pharmaceuticals Ltd, Ahmedabad, Gujarat, India |
II |
Presence of Foreign Tablets/Capsules: Recall is being conducted due to a foreign capsule found in one bottle. |
|
Mercuroclear (benzalkonium chloride 0.13%, lidocaine HCl 2.5%), various pharmacy brands, 1,269,846 bottles |
Humco Holding Group, Inc., Texarkana, TX |
II |
Microbial Contamination of Non-Sterile Products: The product has the potential to be contaminated with Bulkholderia gladioli. |
|
Metronidazole Injection, USP 500mg, 100mL Bag, 567,624 bags |
Hospira, Inc., Lake Forest, IL |
II |
Lack of Assurance of Sterility: There is the potential for the solution to leak from the administrative port to the fill tube seal. |
|
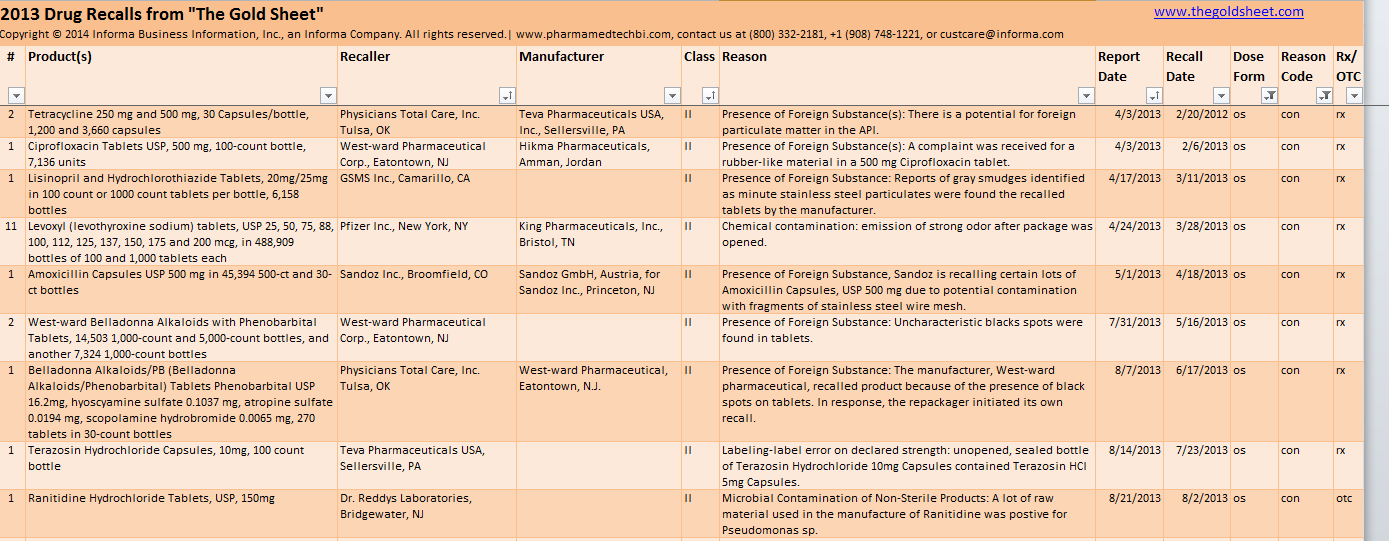
Tetracycline 250 mg and 500 mg, 30 Capsules/bottle, 1,200 and 3,660 capsules |
Physicians Total Care, Inc. Tulsa, OK / Teva Pharmaceuticals USA, Inc., Sellersville, PA |
II |
Presence of Foreign Substance(s): There is a potential for foreign particulate matter in the API. |
|
Ciprofloxacin Tablets USP, 500 mg, 100-count bottle, 7,136 units |
West-ward Pharmaceutical Corp., Eatontown, NJ / Hikma Pharmaceuticals, Amman, Jordan |
II |
Presence of Foreign Substance(s): A complaint was received for a rubber-like material in a 500 mg Ciprofloxacin tablet. |
|
Propofol Injectable Emulsion 1% packaged 5x20 ml and 10x100 ml, 68,020 single patient infusion vials |
Hospira, Inc., Lake Forest, IL |
II |
Presence of Particulate Matter: Visible particulate and particulate embedded in vials were observed during retain inspection. |
|
Amoxicillin for Oral Suspension 125mg/5mL 80mL, 100mL, and 150mL, 4,236 bottles |
Dr. Reddys Laboratories Tennessee LLC, Bristol, TN |
II |
Microbial Contamination of Non-Sterile Products: Suspensions made from these lots of Amoxicillin 125 mg/5mL showed yeast and mold growth at the 14 day time point. |
|
0.9% Sodium Chloride Injection USP, 50mL, 624,240 units |
Hospira, Inc., Lake Forest, IL |
II |
Lack of Assurance of Sterility: The product is being recalled due to the product lot being incorrectly released without meeting product specifications. There is the potential for the solution to leak from the administrative port to the fill tube seal. |
|
Lisinopril and Hydrochlorothiazide Tablets, 20mg/25mg in 100 count or 1000 count tablets per bottle, 6,158 bottles |
GSMS Inc., Camarillo, CA |
II |
Presence of Foreign Substance: Reports of gray smudges identified as minute stainless steel particulates were found the recalled tablets by the manufacturer. |
|
Copaxone (glatiramer acetate injection), 20mg/1mL, packaged in 30 single use Pre-Filled syringes and 33 alcohol preps per box, 6,692 boxes |
Teva Pharmaceuticals USA, Sellersville, PA |
II |
Presence of Foreign Substance: Product is being recalled due to receiving an elevated number of patient complaints related to a visible presence of medical grade silicone oil essential to the functionality of the syringe and plunger stopper system. |
|
Levoxyl (levothyroxine sodium) tablets, USP 25, 50, 75, 88, 100, 112, 125, 137, 150, 175 and 200 mcg, in 488,909 bottles of 100 and 1,000 tablets each |
Pfizer Inc., New York, NY / King Pharmaceuticals, Inc., Bristol, TN |
II |
Chemical contamination: emission of strong odor after package was opened. |
|
Amoxicillin Capsules USP 500 mg in 45,394 500-ct and 30-ct bottles |
Sandoz Inc., Broomfield, CO / Sandoz GmbH, Austria, for Sandoz Inc., Princeton, NJ |
II |
Presence of Foreign Substance, Sandoz is recalling certain lots of Amoxicillin Capsules, USP 500 mg due to potential contamination with fragments of stainless steel wire mesh. |
|
25 injectables recalls *
|
Axium Healthcare Pharmacy dba Balanced Solutions Compounding, Lake Mary, FL |
II |
Lack of Assurance of Sterility: All sterile products compounded, repackaged, and distributed by this compounding pharmacy due to lack of sterility assurance and concerns associated with the quality control processes. |
|
* 1,783 vials of TriMix, Methylprednisolone, Sodium Tetradecyl Sulfate, Betamethasone, Triamcinolone Hexacetonide, Cyanocobalamin, Methylcobalamin and Folic Acid, Progesterone in Olive Oil, Phosphatidylcholine, Comprehensive injection #4- SS varied, Mono-mix, Trimix Customs, BiMix Standard, BiMix Plus, Quadmix Custom, 17 Alpha Hydroxyprogesterone Caproate, Quadmix, DMSO 50% Sterile Bladder Irrigation Solution Injectable, Bacteriostatic Water for Injection 0.9%, Triamcinolone Diacetate, Triamcinolone Acetonide, HCG Lyophylized Powder 10,000 unit, Sodium Tetradecyl Sulfate, BiMIx Customs, Cyclosporin in Corn Oil Injectable Balanced Solutions |
|||
|
Cyclosporin Ophthalmic Balanced Solutions, 11 vials |
Axium Healthcare Pharmacy dba Balanced Solutions Compounding, Lake Mary, FL |
II |
Lack of Assurance of Sterility: All sterile products compounded, repackaged, and distributed by this compounding pharmacy due to lack of sterility assurance and concerns associated with the quality control processes. |
|
Propofol Injectable Emulsion, 1%, 200 mg/20mL, 20 mL, 245,250 single-patient infusion vials |
Hospira Inc., Lake Forest, IL |
II |
Presence of Particulate Matter: A single visible particulate was identified during a retain sample inspection. |
|
Preservative-Free Morphine Sulfate Injection, USP 10mg/10mL, 26,100 vials; Diazepam Injection, USP 5mg/mL, 10mL, Multiple-dose fliptop vial, 172,350 vials |
Hospira Inc., Lake Forest, IL / Hospira Inc., Lake Forest, IL |
II |
Lack of Assurance of Sterility: Loose crimp applied to the fliptop vial. |
|
"Well at Walgreens" Regular Strength Antacid Liquid Mint, 15,976 12-oz and 26-oz bottles |
Tarmac Products, Inc. d.b.a Axara Pharmaceuticals, Miami Gardens, FL |
II |
Microbial Contamination of Non-Sterile Products: Lot in question had an elevated microbial count outside of specifications and E. Coli contamination. |
|
Diazepam Injection, USP 5mg/mL, 10mL, 114,000 Multiple-dose fliptop vials; Furosemide Injection USP, 40 mg/4mL, 259,050 4ml single does vials; Quelicin Injection, USP, 139,200 200 mg 10 mL vials; Sodium Acetate Injection, USP, 40mEq/20ml, 266,900 20mL Single-Dose Fliptop vials. |
Hospira Inc., Lake Forest, IL |
II |
Lack of Assurance of Sterility: Loose crimp applied to the fliptop vial. |
|
Methylcobalamin Injection, 1mg/mL, 32 30mL vials |
NuVision Pharmacy Inc., Dallas, TX |
II |
Lack of Assurance of Sterility; reports of adverse events after injection. |
|
82 injectables recalls *
|
Green Valley Drugs, Henderson, NV |
II |
Lack of Assurance of Sterility: All sterile products compounded, repackaged, and distributed by this compounding pharmacy due to lack of sterility assurance and concerns associated with the quality control processes. |
|
* All known strengths and presentations of: Bacteriostatic Water for Injection, 597 vials ; B-Complex Injection, 679 vials; Betamethasone Combination Injection, 295 vials and syringes; BET-ACE Lido 6-2 Injection including Betameth Combo 6 mg/mL Lidocaine 2 % Syringe, 435 vials and syringes; Betamethasone Sodium Phosphate Injection, 65 vials; Betamethasone Combination with Lidocaine 1% and Bupivacaine 0.5% Injection, 270 syringes; Biotin Injection, 7 vials; Bupivacaine and Dexamethasone Injection, 1,150 vials; Bupivacaine Injection, 756 vials ; Buprenorphine Injection, 2 vials; Vitamin D3 Injection, 18 vials; Cyanocobalamin Injection, 6292 vials; Cyclosporine Injection, 1 vial; Dexamethasone Sodium Phosphate Injection, 4,399 vials; Dexpanthenol Injection, 273 vials; Dimercapto-propane-sulfonic acid injection, 251 vials; Dimethyl Sulfoxide Injection, unknown quantity; Edetate Disodium Injection, 98 vials; Edetate Calcium Disodium Injection, 227 vials; ED Mix Injections, 1,0684 vials; Estradiol Cypionate Injection, 48 vials; Estradiol Valerate Injection, 243 vials; Folic Acid Injection, 244 vials; Furosemide Injection, 227 vials; Gentamicin Injection, 1,140 vials; Glycerin/Lidocaine/Epinephrine Injection, unknown quantity; Human Chorionic Gonadotropin Injection, 5,255 vials and syringes; Hyaluronidase Injection, 996 vials; Hydrochloric Acid Injection, 63 vials; Hydrogen Peroxide Injection, 140 vials; Hydroxocobalamin Injection, 135 vials; Hydroxyprogesterone Caproate Injection, 229 vials; Ketorolac Injection, 3,289 vials; L-Carnitine Injection, 65 vials; L-Glutathione Injection, 430 vials; Lidocaine Injection, 3,946 vials; Lipotropic With Vitamin Injections, 209 vials; Lipotropic With Vitamin with Methylcobalamin Injections, 176 vials; L-Lysine Injection, 132 vials; Magnesium Chloride Injection, 197 vials; Magnesium Sulfate Injection, 529 vials; Manganese Sulfate Injection, 133 vials; Medroxyprogesterone Acetate Injection, 1,299 vials and syringes; Methylcobalamin Injection, 1,343 vials; Methylprednisolone Acetate and Marcaine Injection, 607 vials; Methylprednisolone and Dexamethasone Injection, 430 vials and syringes; Methylprednisolone and Bupivacaine Injection, 52 syringes; Methylprednisolone, Lidocaine, and Sodium Chloride Injection, 720 syringes; Lipotropic With Vitamins, Carnitine, Chromium and Glutamine Injection, 19 vials; Methylprednisolone Acetate Injection, 4,350 vials and syringes; Metoprolol Injection, 65 vials; MIC Vitamin Injection, 588 vials and syringes; Modified Myers Cocktail Injection, 70 vials; Nandrolone Decanoate Injection, 43 vials; Phenylephrine Injection, 54 vials; Phosphatidylcholine and Sodium Deoxycholate Injection, 40 vials; Polidocanol Injection, 109 vials; Procaine Hydrochloride Injection, 70 vials; Progesterone Injection, 13 vials; Pyridoxine Hydrochloride Injection, 319 vials; Sodium Chloride and Dexamethasone Injection, 950 syringes; Sodium Bicarbonate and Lidocaine Injection, 335 vials; Nandrolone Decanoate and Testosterone Cypionate Injection, 5 vials; Sodium Bicarbonate Injection, 6,213 vials; Sodium Chloride Injection, 318 vials; Sodium Tetradecyl Sulfate Injection, 203 vials; Testosterone Cypionate and Testosterone Propionate Injection, 51 vials; Taurine Injection, 50 vials; Testosterone Propionate, Testosterone Cypionate, and Nandrolone Decanoate Injection, 2 vials; Testosterone Cypionate Injection, 3,727 vials and syringes; Thiamine Hydrochloride Injection, 70 vials; Triamcinolone Injection, 1,423 vials; Zinc Sulfate Injection, 208 vials. |
|||
|
35 injectables recalls *
|
Nora Apothecary and Alternative Therapies, Inc., Indianapolis, IN |
II |
Lack of Assurance of Sterility: FDA inspection findings resulted in concerns regarding quality control processes. |
|
* 3 Bimix penile injection formulations; 1 Papaverine penile injection formulation; 4 strengths of PGE-1 penile injection; 5 Trimix strengths for penile injection; 1 Super Trimix strength; Folic Acid injection, 5mg/ml MDV, 10 ml Subcutaneous; HCG Injection, 4 strengths/amounts, subcutaneous; Hydroxycobalamin Injection, 3 strengths/amounts; Methylcobalamin Injection, 5 strengths/amounts; Hydroxyprogesterone Injection, intramuscular; Renacidin bladder irrigation; Gentamicin irrigation; Glutathione injection; M.I.C.+B12 Injection; Lidocaine 2% Injection; Meperidine 100 mg/ml MDV; and Pyridoxine 100mg/ml Injection MDV. Expiration periods ranged from 10 to 90 days, with 90-day expiration the most common, followed by 30-day. Volumes ranged from one to 128 units. |
|||
|
8 Ophthalmologics recalls: Vancomycin Ophthalmic Solution, 3 strengths and amounts; Tobramycin Ophthalmic Solution, two strengths/amounts; as well as Chlorhexidine, Dexamethasone and PHMB Ophthalmic Solutions. |
Nora Apothecary and Alternative Therapies, Inc., Indianapolis, IN |
II |
Lack of Assurance of Sterility: FDA inspection findings resulted in concerns regarding quality control processes. |
|
24 injectables recalls *
|
The Compounding Shop, St. Petersburg, FL |
II |
Lack of Assurance of Sterility: FDA inspection findings resulted in concerns regarding quality control processes. |
|
* One to 80 vials each of Methotrexate 25mg/mL Injectable, 10mL vials; Avastin 1.25mg/0.05 mL Injectable, 4mL vials ; Baclofen 500 mcg/mL Injectable, 22 mL vial; Baclofen 1,000 mcg/mL Injectable, 20 mL and 40 mL vial; Baclofen/Bupivacaine 2,000 mcg/20 mg/mL, 45 mL vial ; Betamethasone 6mg/mL Injectable, 5mL vial ; Dexpanthenol 200 mg/mL Injectable, 100 mL vial ; Dexamethasone 10mg/mL Injectable, 2 mL vial; Hydroxyprogesterone 250 mg/mL Injectable, 4ml, 6ml, 8ml and 10ml vials; Sodium EDTA 150 mg/mL Injectable, 250 mL vial ; Glutathione 200mg/mL Injectable, 50 mL vials ; Lipoic Acid 25 mg/mL Injectable, 100 mL vials ; Mitomycin 0.3 mg/mL Injectable, 10mL vials ; Methylcobalamin 1,000 mcg/mL Injectable, 50 mL vials ; Procaine 1% Injectable, 100 mL and 250 mL vials ; Ascorbic Acid 500 mg/mL Injectable, 100 mL and 500 mL vials ; B-Complex 100 mg/mL Injectable, 50 mL vials; Phosphatidyl Choline 10% Injectable, 500 mL vial ; MIC (methionine, inositol, choline) + B12 25/50/50mg/1000mcg/mL Injectable, 30 mL vials ; Testosterone Cypionate 200 mg/mL Injectable, 10 mL vials ; Morphine Sulfate 25 mg/mL Injectable, 40 mL vial ; Praziquantel 56.8mg, 50 mL vial ; MIC (methionine, inositol, choline) Injectable, 30 mL vials; Tri-Mix Injections (alprostadil, phentolamine, papaverine) in 5 mL and 10 mL vials |
|||
|
Carnosine 5% Opth drop, one 40 mL vial |
The Compounding Shop, St. Petersburg, FL |
II |
Lack of Assurance of Sterility: FDA inspection findings resulted in concerns regarding quality control processes. |
|
Various mixtures of Proplete, Baxter, Fresenius and IDPN intradialytic parenteral nutrition dialysate solution with added amino acids, each involving one to 59 bags |
Pentec Health Inc., Boothwyn, PA |
II |
Lack of Assurance of Sterility: Sterility could not be assured for compounded sterile renal nutritional prescriptions. |
|
DOBUTamine in 5% Dextrose Injection, USP, 250 mg, 1000 mcg, 44,748 bags |
Hospira Inc., Lake Forest, IL |
II |
Lack of Assurance of Sterility: Confirmed report of leaking in the primary container. |
|
Diazepam Injection, USP 5mg/mL, 10mL, 95,700 Multiple-dose fliptop vials |
Hospira Inc., Lake Forest, IL |
II |
Lack of Assurance of Sterility: Loose crimp applied to the fliptop vial. |
|
Cefazolin, injection 1 gram per vial , 297,200 vials |
Sandoz Inc., Broomfield, CO / Sandoz GmbH, Austria |
II |
Lack of Assurance of Sterility due to a customer complaint for broken/cracked vials which was confirmed through review of retained samples. |
|
Cleocin Phosphate (clindamycin Injection), 898,900 vials |
Pharmacia & Upjohn LLC, Kalamazoo, MI |
II |
Presence of Particulate Matter: Firm is recalling a small number of vials with very small reflective flakes consistent with delamination of the glass vial. |
|
0.9% Sodium Chloride Injection, USP 250 mL, 676,872 single-dose flexible plastic containers |
Hospira Inc., Lake Forest, IL |
II |
Lack of Assurance of Sterility: The product has the potential for solution to leak at or near the administrative port of the primary container. |
|
9 injectables recalls *
|
Hospira Inc., Lake Forest, IL |
II |
Lack of Assurance of Sterility: Hospira, Inc is voluntarily recalling the products due to possible leaking bags. |
|
* 0.9% Sodium Chloride Injection, USP, Polyolefin Flexible Containers, 150, 250, 500 and 1,000 mL, 2,137,660 units; 70% Dextrose Injection USP, 2000 mL, 14,706 units; 0.45% Sodium Chloride Injection, USP, 138,584 units; 5% Dextrose Injection USP, 250 and 500 mL, 267,288 units; 5% Dextrose and 0.45% Sodium Chloride Injection, USP 1000 mL VisIV Containers, 74,256 units ; 20% Dextrose Injection, USP, 500 and 1,000 mL, 216,396 units; 0.9% Sodium Chloride Irrigation, USP, 3000 mL , 98,084 units; 1.5% Glycine Irrigation, USP, 3000 mL , 40,988 units; and 0.9% Sodium Chloride Injection, USP, 50 and 100 mL, 2,933,936 units |
|||
|
Acetylcysteine 10% Solution; Acetylcysteine 20% Solution |
Clinical Specialities Compounding Pharmacy, Augusta, GA |
II |
Lack of Assurance of Sterility: FDA inspection findings resulted in conerns regarding quality control processes. |
|
37 injectables recalls *
|
Clinical Specialities Compounding Pharmacy, Augusta, GA / Clinical Specialities Compounding Pharmacy, Augusta, GA |
II |
Lack of Assurance of Sterility: FDA inspection findings resulted in concerns regarding quality control processes. |
|
* Small quantities of Diazepam 5 mg/ml; Estradiol Cypionate 5mg/ml; Estradiol Valerate 20mg/ml; Estradiol Valerate 30mg/ml; Gentamicin buffered 36.4mg/ml; HCG 1000 units/ml, 30mL syringe; HCG 500 units/ml, 10mL syringe; Hydroxyprogesterone 250mg/ml; Injection vehicle, 120ml; Medroxyprogesterone 150mg/ml; Medroxyprogesterone 50mg/ml; Methionine/Choline/Inositol/B-12; Methylcobalamin 1250 mcg/ml; Methylcobalamin 12.5mg/ml; Mitomycin 0.04% solution; Mitomycin 0.02% solution; Pap 300 mg-phent 10mg prost 100mcg; Phenylephrine-cyclopentolate; Progesterone 50mg/ml; Sodium Chloride 23.4%; Test Cyp (testosterone cypionate) 10mg/ml; Test Cyp 100 mg/ml; Test Cyp 200 mg/ml; Test cyp 25mg-Estradiol valerate 15mg/mL; Test cyp 25mg-Estradiol cyp 5mg/ml; Test Cyp 250mg-lidocaine 5mg/ml; Test cyp 25mg-estradiol cyp 7mg/ml; Test Cyp 25mg-e blend 12mg/ml; Test Cyp 35mg/ml; Test Cyp 50mg/ml; Test Cyp 50mg-estradiol cyp 2mg/ml; Test Cyp 50mg-estradiol cyp 7mg/ml; Test Cyp 50mg-estradiol blend 22mg; Test Cyp 75mg-estradiol cyp 5mg/ml; Tetracaine 0.25% solution; Vancomycin 50mg/ml; and 845,520 units of 0.9% Sodium Chloride Injection, USP, in Mini-Bad plus container |
|||
|
Acetylcysteine 2% Solution; Cataract drops; Cyclosporine 1% ophthalmic drops; Cyclosporine 2% ophthalmic; Itraconazole 1% eye ointment; Laser ophthalmic drops, 12ml bottle; Latanoprost 0.025% eye drop; Ophthalmic dilation gel; and Ophthalmic dilation gel |
Clinical Specialities Compounding Pharmacy, Augusta, GA / Clinical Specialities Compounding Pharmacy, Augusta, GA |
II |
Lack of Assurance of Sterility: FDA inspection findings resulted in concerns regarding quality control processes. |
|
0.9% sodium chloride injection, USP, 50 and 100 ml, 730,848 units;5% Dextrose Injection USP in VIAFLEX Plastic Container Multi Pack, 730,848 units; Heparin Sodium and 0.9% Sodium Chloride Injection; Lidocaine Hydrochloride and 5% Dextrose Injection, USP, 2g; Sodium Chloride Injection, USP, in various types of Viaflex packs, 1,788,292 units; and Metronidazole Injection, USP RTU, 500mg/100ml, 172,296 units |
Baxter Healthcare Corp., Deerfield, IL |
II |
Lack of Assurance of Sterility: Specific lot numbers of these products have been identified for potential administration port leakage. The potential for leakage is the result of a manufacturing issue which occurred during the sealing of the closure assembly |
|
Gentamicin Sulfate Injection USP 80mg/2mL , 168,300 single-dose flip-top vials |
Hospira Inc., Lake Forest, IL |
II |
Presence of Particulate Matter: Confirmed customer report where visible particles were identified floating in the primary container. |
|
Benztropine Mesylate Injection, USP, 2mg/2mL, 65,110 single-dose vials |
APP Pharmaceuticals LLC, Schaumburg, IL, and Nexus Pharmaceuticals Inc., Vernon Hills, IL / Fresenius Kabi USA, Lake Zurich, IL |
II |
Presence of particulate matter: characterized as thin colorless flakes that are visually and chemically consistent with glass delaminiation observed in reserve sample vials. |
|
Arm & Hammer Sodium Bicarbonate Powder, USP grades 1-5, in bags of various quantities totalling 749 tons |
Church & Dwight Inc., Princeton, NJ |
II |
Presence of Foreign Substance: raw material recalled due to stainless steel and other contamination. |
|
All Compounded Products, packaged in plastic infusion bags, devices, syringes and glass vials (no quantity given) |
Med Prep Consulting, Tinton Falls, NJ |
II |
Lack of Assurance of Sterility; potential for mold contamination. |
|
VIVITROL (naltrexone for extended-release injectable suspension), 3,325 380mg vials |
Alkermes Inc., Waltham, MA |
II |
Lack of Assurance of Sterility; product did not meet the criteria for container closure integrity testing during routine 24 month stability testing. |
|
West-ward Belladonna Alkaloids with Phenobarbital Tablets, 14,503 1,000-count and 5,000-count bottles, and another 7,324 1,000-count bottles |
West-ward Pharmaceutical Corp., Eatontown, NJ |
II |
Presence of Foreign Substance: Uncharacteristic blacks spots were found in tablets. |
|
AmBisome (amphotericin B) liposome for Injection, 133,550 50mg single-use vials |
Astellas Pharma US, Northbrook, IL / Gilead Sciences Inc., San Dimas, CA |
II |
Lack of Assurance of Sterility; Astellas Pharma US, Inc. is performing a voluntary recall on certain lots of AmBisome because the manufacturer has notified Astellas that during a routine simulation of the manufacturing of AmBisome, a bacterial contamination was detected in the media fills. |
|
AmBisome (amphotericin B) liposome for Injection, 478,352 50mg single-use vials |
Gilead Sciences, Inc., Foster City, CA / Gilead Sciences Inc., San Dimas, CA |
II |
Lack of Sterility Assurance; During a routine simulation of the manufacturing of AmBisome, a bacterial contaminiation was detected in some media fill units. No contaminated batches have actually been identified in the finished product, but there is a possibility of contamination. |
|
Sodium Chloride Injection, USP, 0.9%, 268,700 20mL Single-dose Fliptop Plastic Vials |
Hospira Inc., Lake Forest, IL |
II |
Presence of Particulate Matter: Confirmed customer report of visible particulate in the form of an orange or rust colored ring embedded in between the plastic layers of the plastic vial. |
|
Belladonna Alkaloids/PB (Belladonna Alkaloids/Phenobarbital) Tablets Phenobarbital USP 16.2mg, hyoscyamine sulfate 0.1037 mg, atropine sulfate 0.0194 mg, scopolamine hydrobromide 0.0065 mg, 270 tablets in 30-count bottles |
Physicians Total Care, Inc. Tulsa, OK / West-ward Pharmaceutical, Eatontown, N.J. |
II |
Presence of Foreign Substance: The manufacturer, West-ward pharmaceutical, recalled product because of the presence of black spots on tablets. In response, the repackager initiated its own recall. |
|
Ondansetron Injection, USP, 4mg/2ml, 340,600 single-dose fliptop vials |
Hospira Inc., Lake Forest, IL |
II |
Presence of Particulate Matter; lot being recalled as a precaution due to the discovery of 2 particles found in a lot which preceded the recalled lot. |
|
Terazosin Hydrochloride Capsules, 10mg, 100 count bottle |
Teva Pharmaceuticals USA, Sellersville, PA |
II |
Labeling-label error on declared strength: unopened, sealed bottle of Terazosin Hydrochloride 10mg Capsules contained Terazosin HCI 5mg Capsules. |
|
Latanoprost Ophthalmic Solution, 0.0005%, 2.5mL bottle, 50,984 bottles |
Apotex Inc., Richmond Hill, CA / Apotex Inc., Toronto, Ontario, Canada |
II |
Lack of Assurance of Sterility: Failed at expiry for Preservative Effectiveness Test(PET),therefore the product may be susceptible to microbial growth before the expiry date. |
|
20 injectables recalls *
|
Pallimed Solutions Pharmacy, Woburn, MA |
II |
Lack of Assurance of Sterility: FDA inspectional findings resulted in concerns associated with quality control procedures that impacted sterility assurance. |
|
* Alprostadil IN NS Injection, 408 vials; Atropine Injection; Bacteriostatic Water for Injection; BiMix Injection, 246 vials; Buprenorphine HCI Veterinary Injection, 71 vials; Diazepam Injectable, 7 vials; Dexamethasone PF; DMSO Aqueous Irrigation 50%, 3 vials; Gentamicin Sulfate Irrigation; HCG Chorionic Gonadotropin, 261 vials; Hydroxyprogesterone caproate injection; Methylcobalamin PF, 3 vials; MIC with B6 and B12, 18 vials; Nandrolone Decanoate Injectable, 56 vials; Quadmix Injection, 2 vials; Testosterone Cypionate/Testosterone Enanthate Injection; Testosterone Cypionate/Propionate Injection, 10 vials; Testosterone Cypionate Injection, 597 vials; Trimix Injection, 862 vials; and Verapamil Injection |
|||
|
Acetylcysteine Ophthalmic Solution, 1 vial; Cidofovir Opthalmic Solution, 23 vials; Cyclosporine Ophthalmic; Tacrolimus Ophthalmic; and Vancomycin Ophthalmic PF, 12 vials |
Pallimed Solutions Pharmacy, Woburn, MA |
II |
Lack of Assurance of Sterility: FDA inspectional findings resulted in concerns associated with quality control procedures that impacted sterility assurance. |
|
Propofol Injectable Emulsion, 10mg/mL, 100mL and 500mL, 94,110 single-patient infusion vials |
Hospira Inc., Lake Forest, IL |
II |
Presence of Particulate Matter; single visible particulate was identified during a retain sample inspection identified as stainless steel. |
|
Belladonna Alkaloids with Phenobarbital Tablets, 61,691 1,000-count and 5,000-count tablets |
West-ward Pharmaceutical Corp., Eatontown, NJ |
II |
Presence of Foreign Substance: black specks comprised of degraded organic material found on tablets. |
|
Gastrografin (diatrizoate meglumine and diatrizoate sodium solution) USP, 37% Organically Bound Iodine, 29,436 120ml bottles |
Bracco Diagnostics Inc., Monroe Township, NJ / Therapex, Division of E-Z-EM Canada Inc., Anjou, Quebec, Canada |
II |
Presence of foreign substance: one lot of the product may contain black foreign particles. |
|
Ranitidine Hydrochloride Tablets, USP, 150mg |
Dr. Reddys Laboratories, Bridgewater, NJ |
II |
Microbial Contamination of Non-Sterile Products: A lot of raw material used in the manufacture of Ranitidine was postive for Pseudomonas sp. |
|
REEVA Antibacterial Hand Soap and Dishwashing Liquid, 0.10%, 11,520 24oz bottles |
Showline Automotive Products, Raleigh, NC |
II |
Microbial Contaimination of Non-Sterile Product was found to be contaminated with the bacteria, Sarcina Lutea. |
|
Eyebright Concentrate Topical Eye Rinse 1, 2, 4, 8, 16 & 32 fl oz, 868 bottles |
Earthlabs, Inc. DBA Wise Women Herbals, Creswell, OR |
II |
Lack of Assurance of Sterility; Lack of Assurance of Sterility; product not manufactured under sterile conditions as required for opthalmic drug products. |
|
Avastin (0.05ml, 1.25mg/0.05ml) 2,190 single unit dose syringes |
Clinical Specialities Compounding Pharmacy, Augusta, GA |
II |
Lack of Assurance of Sterility; product linked to adverse event reports or endophthalimitis eye infections and FDA inspection findings resulted in concerns regarding quality control processes. |
|
Benztropine Mesylate Injection, USP, 2mg/2mL, 46,185 Single-Dose Vials |
Nexus Pharmaceuticals Inc., Lincolnshire, IL / Nexus Pharmaceuticals Inc., Vernon Hills, IL |
II |
Presence of particulate matter: characterized as thin colorless flakes that are visually and chemically consistent with glass delamination. |
|
Ketorolac Tromethamine Inj., USP, 345,800 30mg vials |
Hospira Inc., Lake Forest, IL |
II |
Lack of Assurance of Sterility: Either a loose crimp or no crimp was applied to the fliptop vials. |
|
Diazepam Injection, USP 5mg/mL, 72,300 10mL Multiple-dose fliptop vials |
Hospira Inc., Lake Forest, IL |
II |
Lack of Assurance of sterility: ineffective crimp on fliptop vials that may result in leaking at the neck of the vials. |
|
Carboplatin injection, 600mg/60ml, 780 Multidose vials |
Teva Pharmaceuticals USA, Sellersville, PA / Teva Parenteral Medicines, Irvine, CA |
II |
Lack of Assurance of Sterility: The required reduction of endotoxin was not met during the annual revalidation of the vial washer. |
|
85 injectables recalls *
|
Beacon Hill Medical Pharmacy, Southfield, MI |
II |
Lack of Assurance of Sterility: FDA inspectional findings resulted in concerns associated with quality control procedures that impacted sterility assurance. |
|
* Sodium Phosphate Dibasic/water for injection 10% all presentations; Sodium Phosphate Monobasic/water for injection 10% all presentations; Testosterone Hypoallergenic 200mg/ml injectable and Testosterone Aqueous 100mg/ml injectable; Testosterone Cypionate 100mg/ml, 200mg/ml, 20mg/ml Oil ; Testosterone Propionate 100mg/ml for injection; Tri-Mix 10MCG/30 MG/1 MG/ML Inj Soln and Tri-Mix 30 MCG/15 MG/2 MG/ML INJ SOLN; Triamicinolone Acetonide 80mg/ml Inj SUSP, all configurations; Triple-Testosterone 250 MG/ML Oil Inj SOLN and Triple-Testerone 200 MG/ML Oil INJ SOLN ; Tripitropin Plus Injectable, all configurations ; Vitamin B-Complex Injectable, Vitamin B-Complex SL SOLN and Vitamin B-Complex Injectable ; Vitamin D 50,000 LU./ML Injectable ; Zinc Sulfate 1mg/ml Injectable ; Alprostadil 100 MCG/ML Inj SOLN, all sizes; Ascorbic Acid 500 mg/ml Injectable ; B-12 Rejuv 25 mg/ml Injectable ; Calcium Edta 200 mg/ml injectable ; Calcium EDTA 300mg/ml injectable ; Manganese Sulfate 0.1 mg/ml Injectable ; Acetylcysteine 100 mg/ml INJ SOLN ; Ascorbic Acid 500mg/ml Injectable ; B-12/LIDOCAINE 25/10MG/ML injectable; B-Complex/Lido/B-12, 25mg/ml injectable ; Alprostadil, 50 mcg/ml, INJ SOLN; Arginne (r-gene) 100 mg/ml injectable ; B-Complex/Lidocaine 2% Injection ; B12/E2/E3/PROG/Test 1/3.75/2.5/50/45 mg/ml inj solution; BI-MIX Phentolamine/Papaverine 0.5 mg/30 mg/ml and Bi-Mix Phentolamine/Papaverine 1 mg/30 mg/ml Injectable; Bi-Tropin S-2 Injectable ; Calcium Chloride 100mg/ml Injectable ; Chromium 4 mcg/ml injectable ; Copper 0.4 mg/ml injectable ; Cyanocobalamin 1000 mcg/ml INJ SOLN ; Dexamethasone Sodium Phosphate 10mg/ml Injectable ; Dexapanthenol 250 mg/ml Injectable ; Dextrose PF 50% Injectable ; DMSO 50% INJ 50 ML Vial Injectable ; DMPS 50mg/ml Injectable ; Dual Testosterone 20,50,100,150, 200 mg/ml Oil INJ SOLN ; Edetate Disodium 150 mg/ml Injectable ; Estradiol Cyp/Prog/Test Cyp 0.5/10/10 mg/ml oil injection solution; Estradiol Cypionate 5,10, 40 mg/ml Oil INJ SOLN; Estradiol Valerate w/Lidocaine 1% 40mg/ml Oil INJ SOLN ; Estradiol 5 mg/ml Oil INJ SOLN ; Freamine III ALT 8.5% Injectable ; Furosemide 10mg/ml injectable ; Glutamine 100 mg/ml INJ SOLN ; Glutathione 200 mg/ml INJ SOLN ; Glutathione/Lido 100mg/2% ml INJ SOLN ; Glutathione/Methyl/Lido 100/25/20 mg/ml INJ SOLN and Glutathione/Methyl/Lido 100/25/20 mg/ml ; Glycine 50 mg/ml Injectable; Glycyrrhizic Acid 8mg/ml Injectable ; H.C.G 5,000, 8,000 , 10,000, 11,000 and 12,000 unit vials injectable ; Hydrogen Peroxide 3% Injectable ; Hyaluronidase 150U/ML and 200 U/ML Injectable; Hydroxocobalamin 1MG/ML Injectable ; Hydroxyprogesterone Caproate 250 mg/ml Oil injection solution; Ketorolac Tromethamine 60mg/ml Injectable ; L-Tryptophan 300 mg/ml Injectable ; Lidocaine MDV 1% Injectable, Lidocaine MDV 20mg/ml Injectable and Lidocaine PF 20mg/ml injectable; Lipo-Plex Injectable ; Lipoic Acid 100 mg/ml and 200 mg/ml Injectable ; Magnesium Chloride Hexahydrate 20% Injectable ; Magnesium Sulfate 50% Injectable ; Methylcobalamin 1 mg/ml, 5mg/ml , 10mg/ml, 25 mg/ml and Methylcobalamin 1mg/ml, 25mg/ml for injection; Methylprednisolone Acetate 40mg/ml and 80 mg/ml Injectable ; MIC-CARN + B6/B12/Lidocaine 25/50/50/175mcg/1/0.1mg/ml Injectable ; MIC-CARN Max 15/100/50/1 MG/ML Injectable ; Multitrace-5 Injectable ; Nalbuphine HCL 20 mg/ml Injectable ; Naltrexone 1 mg/ml Injectable and 200mg/ml Injectable ; Nandrolone Decanoate 100 mg/ml and 200 mg/ml Oil injection solution; Oxytocin 10 units/ml for injection; Paraben Water for Injection ; Phosphatidylcholine 50mg/ml Injectable ; Potassium Chloride 2 MEQ/ML Injectable ; Procaine P/F 20 mg/ml Injectable ; Prog/Test Cyp 15/7.5 mg/ml Oil for injection; Progesterone 50mg/ml Oil injection solution; Pumice/Lidocaine HCL 1.5%/ 1% INJ SUSP; Pyridoxine HCL P/F 100mg/ml Injectable ; Quad Amino Blend 150/75/150/75 mg/ml Injectable ; Selenium 400 mcg/ml Injectable ; Sermorelin Acetate 10mg injectable ; Sodium Bicarbonate PF 8.4% injectable ; and Sodium Hydroxide 10% water for Injection. |
|||
|
Buprenorphine 0.1/0.05 mg/ml Cream |
Beacon Hill Medical Pharmacy, Southfield, MI / Beacon Hill Medical Pharmacy, Southfield, MI |
II |
Lack of Assurance of Sterility: FDA inspectional findings resulted in concerns associated with quality control procedures that impacted sterility assurance. |
|
Methylcobalamin Injection 5mg/ml, 297 30mL multi-dose vials; Multitrace-5 Concentrate Solution (trace elements), 82 10mL multi-dose vials for slow IV administration after dilution; Testosterone Cypionate (Sesame Oil) 200mg/ml, for injection in 10mL amber vials |
South Coast Specialty Compounding, Irvine, CA / Park Compounding, Irvine, CA |
II |
Lack of Assurance of Sterility: Park Compounding is recalling Methylcobalamin 5mg/mL, Multitrace-5 concentrate, and testosterone cypionate (sesame oil) for injection due to lack of sterility assurance. |
|
Glutathione 100mg/ml Injectable, 16 100ml vials |
Creative Compounds, Wilsonville, OR |
II |
Glutathione 100mg/mL injectable human drug is recalled due to Out of Specification results or potential bacterial contamination and it was reported as passing by a contract laboratory. |
|
Lidocaine 1% PF Sterile Injection, 30mL Single Dose Vial, 50 vials; EDTA disodium 150 mg/mL Sterile Injection, 75 20mL multi-dose vials |
Pharmacy Creations, Randolph, NJ / Pharmacy Creations, Randolph, NJ |
II |
Lack of Assurance of Sterility: Pharmacy Creations is recalling Lidocaine 1% PF Sterile Injection and EDTA disodium 150mg/ml due to lack of assurance of sterility. |
|
Propfol Injectable Emulsion, 1%, 200mg/20mL. 283,150 single-patient infusion vials |
Hospira Inc., Lake Forest, IL |
II |
Presence of Particulate Matter: Visible particulate and particulate embedded in vials were observed during retain inspection. |
|
Dexapanthenol 250mg/ml PF, 1 mL, 1,355 units; Magnesuim Sulfate Hepta 50% PF, 590 2mL and 10mL Single Use Vials; Methyl B12 1mg/ml, 2,687 1mL and 10mL Single-Use Vials; NA. Phenylbutyrate 200mg/ml, 200 10mL, 25mL and 50mL single-dose vials; RL Glutathione 100mg/ml, 34 single-dose vials, 1, 2,3 and 4 mL; Dexpanthenol 250mg/ml, 1,355 1mL single-use vials |
Wellness Pharmacy, Birmingham, AL |
II |
The product lots are being recalled due to laboratory results indicating microbial contamination. The FDA was concerned test results obtained from the recalling firm's contract testing lab may not be reliable. |
|
Testosterone Cypionate (Sesame Oil) 200mg/ml, 178.2 mL in 10mL vials; Testosterone CYP (Sesame Oil) 200mg/ml, 965.50 mL in 10mL vials; Testosterone Cypionate/Testosterone Propionate 180-20 mg/mL injection, seven 10mL vials; PGE-1, 20mcg/mL Injection in Normal Saline, 85 mL in 10mL vials; and PGE-1, 100 mcg/ml Injection in Normal Saline , 37,805 mL I 50mL vials |
University Compounding Pharmacy, San Diego,CA |
II |
Lack of Assurance of Sterility: University Compounding Pharmacy is volunatrily recalling certain pharmacy products due to lack of assurance of sterility concerns. |
|
Acetylcysteine 20% Solution Preservative Free, 4mL Sterile Single Dose Vial, not for injection, 903 vials |
JCB Labs LLC, Wichita, KS / JCB Laboratories, Wichita, KS |
II |
Lack of Assurance of Sterility: The product lots are being recalled due to laboratory results indicating microbial contamination. The FDA was concerned test results obtained from the recalling firm's contract testing lab my not be reliable. |
|
Sodium Thiosulfate 25% 250 mg/mL sterile solution for injection, 1,056 50mL single-dose vials; Sodium Citrate 4% containing Gentamicin 320 mcg/ml, 397 30mL multidose vials for injection; Sodium Citrate 4% Solution for Injection, 677 30mL multi-dose vials; and Epinephrine 1:1,000 Sterile Solution for injection, preservative-free and sulfite-free, 1,243 1mL single-use syringes |
JCB Labs LLC, Wichita, KS / JCB Laboratories, Wichita, KS |
II |
Lack of Assurance of Sterility: The product lots are being recalled due to laboratory results indicating microbial contamination. The FDA was concerned test results obtained from the recalling firm's contract testing lab my not be reliable. |
|
Dextrose 50% Preservative Free SDV Injection Solution, 50mL Single dose, 56 vials; Hyaluron (hyaluronidase) 150 units/mL preserved solution, 810 1mL multi-dose vials ; and Lidocaine/Phenylephrine PF 1%/1.5% injectable, 728 1mL single-use vials |
Leiter's Pharmacy, San Jose, CA |
II |
Lack of Assurance of Sterility: Leiter Pharmacy is recalling due bacterial contamination found after investigation at contract testing laboratory discovered that OOS results were reported to customers as passing. Hence the sterility of these products cannot be assured. |
|
Bevacizumab (Avastin) 2.5mg/0.1mL preservative-free injection, 0.1mL syringe, 265 vials |
Leiter's Pharmacy, San Jose, CA |
II |
Lack of Assurance of Sterility: Leiter Pharmacy is recalling due bacterial contamination found after investigation at contract testing laboratory discovered that OOS results were reported to customers as passing. Hence the sterility of these products cannot be assured. |
|
1,198 mL of Testosterone Cyp 200 mg/mL, W/Pres. (Benzyl ETOH), 3 mL; Lipo-Injection w/ Lidocaine (PF), Ascorbic Acid 50 mg, B1-50 mg, B2-5-PO4-5 mg, B3-50 mg, B5-5 mg, B6-5 mg, Cyano B12-100 mcg, Methionine 12.5 mg, Inositol-25 mg, Choline-25 mg, Lidocaine 10 mg/mL, 30 mL, 19,803 mL in all; Taurine 50 mg/mL PF, 1,660 mL of 100mL doses; 6,130 mL of L-Glutathione 200 mg/mL PF, 100 mL; 2,790 mL of Pyridoxine HCL 100 mg/mL NS (PF), 30 mL; 1,880 mL of Magnesium Chl 200 mg/mL PF, 100 mL; 15,500 mL of NA Ascorbate 500 mg/mL, PF, 100 mL;and 8,750 mL of NA Ascorbate 500 mg/mL, non corn PF, 100ML |
Medaus Inc. pharmacy, Birmingham, AL |
II |
Lack of Assurance of Sterility: The product lots are being recalled due to laboratory results (from a contract lab) indicating microbial contamination. The FDA was concerned test results obtained from the recalling firm's contract testing lab may not be reliable. Hence the sterility of the products cannot be assured. |
|
Hydroxocobalamin MDV 5 mg/mL (5,000 mcg/mL) Inj., 10 mL vial X 3 |
Northern New England Compounding Pharmacy LLC, Littleton, NH |
II |
Lack of Assurance of Sterility: The pharmacy is recalling one lot of Hydroxocobalamin MDV 5mg/mL due to failed sterility results by a third party contract testing lab. Hence the sterility of the product cannot be assured. |
|
Bevacizumab 1.25 mg/0.05 mL PF |
Avella of Deer Valley Inc., Phoenix, AZ |
II |
Lack of Assurance of Sterility: Avella Specialty Pharmacy is recalling bevacizumab and vancomycin due to concerns of sterility assurance with the specialty pharmacy's independent testing laboratory. |
|
Vancomycin PF (BSS) 1%, 1 mL, 250 units |
Avella of Deer Valley Inc., Phoenix, AZ |
II |
Lack of Assurance of Sterility: Avella Specialty Pharmacy is recalling bevacizumab and vancomycin due to concerns of sterility assurance with the specialty pharmacy's independent testing laboratory. |
|
Walgreens Progesterone in Ethyl Oleate 50mg per mL, intramuscular injection, 298 vials |
Walgreens Co., Deerfield, IL / Walgreens Specialty Pharmacy, Fresco, TX |
II |
Lack of Assurance of Sterility: Walgreens Specialty Pharmacy is recalling one lot of Progesterone in Ethyl Oleate sterile injection due to concerns of sterility assurance with the specailty pharmacy's independent testing laboratory. |
|
5% Lidocaine HCI and 7.5% Dextrose Injection USP, 86,400 2mL single-dose ampules |
Hospira Inc., Lake Forest, IL |
II |
Presence of Particulate Matter; report of glass particles in the ampule after dilution. |
|
Estradiol 1.25mg, Progesterone 25 mg/mL injectable, 3,540 mL of 20mL sterile vials; and SIH-Testosterone Cypionate 200mg/mL Injectable, 1,430 mL of 10mL sterile vials |
The Apothecary Shoppe, Tulsa, OK |
II |
Lack of Assurance of Sterility: concerns of sterility assurance with the pharmacy's independent testing laboratory. |
|
Oxycodone and Acetaminophen Tablets, USP 10mg/650mg, Class II, 10,615 100-count bottles |
Watson Laboratories, Inc., Corona, CA |
II |
Presence of Foreign Tablets/Capsules: 20 tablets of Oxycodone/APAP 7.5/500 mg were found in a sealed 100 count bottle of Oxycodone and Acetaminophen Tablets, USP 10/650mg lot 705791A. |
|
Sodium Phosphate Injectable, 150mm/50mL Phosphorus 200mEq/50mL , 54 50mL single-dose vials; Histamine, 10 mg/mL Injectable, eight multi-dose vials |
Triangle Compounding Pharmacy, Cary, NC |
II |
Lack of Assurance of Sterility: The product lots are being recalled due to laboratory results (from a contract lab) indicating microbial contamination. The FDA was concerned test results obtained from the recalling firm's contract testing lab may not be r |
|
0.5% Bupivacaine HCI and Epinephrine 1:200,000 Injectable, 187,150 10mL single-dose vials |
Hospira Inc., Lake Forest, IL |
II |
Lack of Assurance of Sterility; potential for vial breakage. |
|
Concentrated Motrin infants' Drops Oral Suspension, 197,532 Original Berry Flavor 1/2 fl oz bottles |
McNeil Consumer Healthcare, Fort Washington, PA |
II |
Presence of Foreign Substance: process-related particulates which may be associated with the raw materials were observed. |
|
Albuterol Sulfate Inhalation Solution, 0.083% 2.5mg/3mL for Oral Inhalation Only, 689,568 cartons, 25 3mL sterile unit-dose vials per carton |
Nephron Pharmaceuticals Corp., Orlando, FL |
II |
Lack of Assurance of Sterility: Nephron Pharmaceuticals Corporation conducted a routine periodic aseptic process simulation and discovered bacterial growth in a number of media fill vials, exceeding the allowable limit. |
|
Bevacizumab 25mg/mL, 241 0.5mL and 0.6mL prefilled syringes |
Fallon Wellness Pharmacy, Latham, NY |
II |
Lack of Assurance of Sterility: Fallon Pharmacy recalled Bevacizumab 25mg/mL due to sterility assurance concerns based on testing of this lot by a third party lab, indicating that test results reported as passing sterility may have been inaccurate. |
|
Prolia (denosumab) Injection; 60mg/mL, 4,163 60mg Single-Use Prefilled Syringes |
Amgen Inc., Thousand Oaks, CA |
II |
Presence of Particulate Matter: Visible cellulose fibers were observed in a small number of prefilled syringes during a routine quality examination. |
|
Ropinirole, extended-release tablets, 2mg, 692 90-count bottles |
Sandoz Inc., Princeton, NJ |
II |
Cross contamination with other products: Sandoz is recalling certain lots of Ropinirole Extended Release Tablets 2mg due to the potential presence of carryover coming from the previously manufactured product, mycophenolate mofetil. |
|
Propofol Injectable Emulsion 1% 200mg/20mL, 70,450 20mL single patient infusion vials |
Hospira Inc., Lake Forest, IL |
II |
Presence of Particulate Matter: Visible particles embedded in the glass identified during a retain sample inspection. |
|
Metoclopramide Injection, USP 10mg, 251,400 2ml single-dose vials; and Ondansetron Injection, USP 4mg/2mL, 681,400 2mL Single-dose vials |
Hospira Inc., Lake Forest, IL |
II |
Presence of Particulate Matter: Potential vendor glass issue-glass spiticules were identified during site inspection of the vials. |
|
Copaxone (glatiramer acetate injection) 20mg/1mL, 6,803 cartons of 1mL single-use prefilled syringes, 30/carton |
Teva Pharmaceuticals USA, Horsham, PA |
II |
Presence of Particulate Matter: A foreign particle found in a pre-filled syringe was reported through a consumer complaint about a pre-filled syringe. |
|
Acyclovir Tablets, USP 800mg, 4,844 bottles |
Apotex Inc., Toronto, Ontario, Canada |
II |
Presence of Particulate Matter: Product from one lot may contain tablets with pieces of nitrile rubber glove embdedded within tablets. |
|
298 recalls covering all products distributed prior to 05/23/2013 *
|
Main Street Family Pharmacy, Newbern, TN |
II |
The firm received seven reports of adverse reactions in the form of skin abscesses potentially linked to compound preservative-free methylprednisolone 80mg/ml 10ml vials. |
|
* 17-P 1ml; Acetylcystein 10% 120ml; Acetylcystein 10% 100mg/ml 4ml; Acetylcystein 20% 90ml; Adenosine 35mg/ml, 30ml; Adenosine Monophosphate, 50mg/ml 30ml; Alphahydroxyprogesterone, 250mg; Alprostadil 100mcg/ml 10ml; Alprostadil 20mcg/ml 25ml; Alprostadil 40mg; AMC 12 with lido 10ml; AMC 12 w/out lido 10ml; Amikacin 250mg/ml 2ml; Amikacin 250mg/ml 2ml vial; Amikacin 500mg 2ml; Aminophylline 250mg 10ml; Aminophylline 25 mg/ml 10ml; Ascorbic Acid 500mg/ml 50ml; B-12 Compounded; B-12 (Methyl) 30ml; B-12 0.5ml Syringe; B-12 1ml pre-filled syringe; B-12 mixture; B-12 PFS; B-12/ Adenosine 10ml; Bacteriostatic Water 30ml; B-Complex 100 30ml; B-Complex 206 mg/ml 30ml; Betamethasone Na phosphate 4mg/ml 10ml; Betamethasone 6.5mg 10ml; Betamethasone Na phosphate ; Betamethasone 6mg 10ml; Betamethasone 6mg 10ml PF; Betamethasone 6mg 30ml; Betamethasone 6mg PF 10ml; Betamethasone 7mg; Betamethasone 9mg; Betamethasone 9mg/ml PF; Betamethasone 10mg 10ml; Bi-Mix Papaverine 30 mg Phentolamine 1mg 10ml; Brompheniramine 10ml; Calcium Gluconate 10% 50mL; Calcium Gluconate 2.5% Gel; Carnitine 250mg/ml; Cyclosporine Drops; Depo-Testadiiol; Dexamethasone LA 16mg/ml 10ml; Dexamethasone LA 16mg/ml 30ml; Dexamethasone 4/8; Dexamethasone 8/4 10ml and 30ml; Dexamethasone 8mg 10ml; Dexamethasone LA 16mg; Dexamethasone LA 8mg/ml 10ml; Dexamethasone LA 16mg 30ml; Dexamethasone sodium phosphate; Double Strength PGE1 40mcg 2ml; Double Strength PGE1 40mcg/ml; Double Therapy Injectables SS ML; EDTA 150mg/ml 100ml; EDTA 3% 30ml; Estradiol Valerate 40mg; Estradiol Pellet 20mg; Estradiol Pellet 37.5mg; Estradiol Pellet 50mg; Estradiol Pellets 10mg; Estradiol Pellets 12.5mg; Estradiol Pellets 12mg; Estradiol Pellets 15mg; Estradiol Pellets 25mg; Estradiol Pellets 31 mg; Estradiol Pellets 6mg; G-A-C Amino Cocktail 30ml; Glutathione "Reduced" 200mg/ml; Glutathione 150mg/ml 30ml; Glycerin 72% 30ml; Glycerin 72%, Lido 1% w/Epi 2; Glycolic 70% ph 1.8 gel; Gomenol 10 ml; HCG 100 iu prefilled syringes; HCG 10000u; HCG 1000iu PFS; HCG 11,000U; HCG 11000 w/B-12; HCG 12000u; HCG 140 iu prefilled syringes; HCG 15,000u; HCG 15000u w/b-12; HCG 1500iu PFS; HCG 175 iu prefilled syringes; HCG 180 iu prefilled syringes; HCG 20,000u; HCG 200iu prefilled syringes; HCG 2000IU; HCG 2000iu PFS; HCG 210 iu prefilled syringes; HCG 220 iu prefilled syringes; HCG 220 iu prefilled syringes; HCG 230 iu prefilled syringes; HCG 240 iu prefilled syringes; HCG 250 iu prefilled syringes; HCG 2500u; HCG 260 iu prefilled syringes; HCG 270 iu prefilled syringes; HCG 275 iu prefilled syringes; HCG 280 iu prefilled syringes; HCG 290 iu prefilled syringes; HCG 300 iu prefilled syringes; HCG 3000IU; HCG 310 iu prefilled syringes; HCG 320 iu prefilled syringes; HCG 330 iu prefilled syringes; HCG 340 iu prefilled syringes; HCG 350 iu prefilled syringes; HCG 360iu PFS; HCG 370iu prefilled syringes; HCG 380iu prefilled syringes; HCG 390iu prefilled syringes; HCG 40,000u; HCG 400 iu prefilled syringes; HCG 450 iu prefilled syringes; HCG 5,000U; HCG 500iu; HCG 5000iu kit; HCG 500u/ml w/methylc; HCG 60 iu prefilled syringes; HCG 7000u; HCG Diet Kit; HCG Nasal Spray; Hydrogen Peroxide 3% 30ml; L-Arginine 100mg/ml 30ml; L-Carnitine 30ml; Let Gel,; L-Glutamine 30mg/30ml vial; L-Glutamine 200mg/ml 30ml; Lido 2% MPF 2ml; Lido 4% Phenyl 1%; Lidocaine 1% 30ml; Lidocaine 1% 30ml; Lidocaine 2% 30ml; Lidocaine 4% Phenylephrine 1%; Lincomycin 300 PF 10ml; Lincomycin 300 w/lido 10ml; Lincomycin 300 mg; Lipo B; Lipo B Complex 30ml; Lipo Blast 50ml, Methionine 25mg, Inositol 50 mg, Choline 100mg, Methyl B-12.25mg, B6 25mg, B5 12.5 mg, Lidocaine 10mg, B7 5mg, adenosine 12.5mg, B1 12.5mg, B2 2.5mg, B3 12.5mg, Benzyl Alcohol .02ml 50mlMain; Lipo Blast w/o adenosine, Methionine 25mg, Inositiol 50mg, Choline 100mg, Methyl B-12.25mg, B6 25mg, B6 25mg, B5 12.5 mg, Lidocaine 10mg, B7 5mg, B1 12.5mg, B2 2.5mg, B3 12.5mg, Benzyl Alcohol .02ml 50ml; Lipo C 30ml, Methionine 12.5mg, Inositol 50mg, Choline 100mg, Methylcobalamin 1mg, L-Carnitine 200mg; Lipo X 50ml; Lipoden 50ml; Lipotonix Plus 10ml; Lipotonix Plus 30ml; Lipotonix Plus 30ml w/o methion; Lipotonix Plus w/Proc/Chrom; Lipotonix Plus-Mic B Complex; Lipotropic (Getwell); Lipotropic 1.5ml PFS; Lipotropic 10ml; Lipotropic 10ml with B-12 and L-Carnitine; Lipotropic 1ml PFS; Lipotropic 2ml PFS; Lipotropic 30ml; Lipotropic 50ml; Lipotropic 50ml w/L-Carnitine; Lipotropic 50ml w/o methionine; Male HCG Pk; Male HCG Pk 2nd Month; Male HCG Pk 3rd month; Marcaine .1% NS 300ml pump; Marcaine .1% NS 400ml pump; Marcaine .1% NS 450ml; Marcaine .2% NS 300ml; Marcaine .2% NS 450ml; Medroxyprogesterone 150mg PF/1ml; Medroxyprogesterone 150mg w/lido 10ml; Medroxyprogesterone 150mg 10ml; Medroxyprogesterone 150mg 1ml; Medroxyprogesterone (PF) 150mg/10ml; Medroxyprogesterone 150mg w/lid, 1ml; Mesotherapy; Methylcob 100mcg/ml 30ml; Methylcobalamin 1000mcg/ml 30ml; Methylcobalamin 1 mg/ml 50ml; Methylcobalamin 1mg/ml 30ml; Methylcobalamin 30ml; Methylprednisolone 100mg/ml , 10ml; Methylprednisolone 100mg w/lidocaine; Methylprednisolone 20mg PF 5ml; Methylprednisolone 40 w/lidocaine 10ml; Methylprednisolone 40mg 10ml; Methylprednisolone 40mg PF 10ml; Methylprednisolone 40mg PF 1ml; Methylprednisolone 40mg PF 2ml; Methylprednisolone 40mg w/lidocaine 1ml; Methyprednisolone 40mg, 10ml; Methylprednisolone 80mg/ml 10ml; Methylprednisolone 80mg SDV, 10ml; Methylprednisolone 80mg 10ml; Methylprednisolone 80mg PF 1ml; Methylprednisolone 80mg w/lidocaine 10ml; Methylprednisolone Acetate 80mg 1ml; Mic .05ml syringe; Mic 25/50/50 30ml; Mic 25/50/50 50ml; Mic 25/50/50 w/333mg B-12; Mic 30ml; Mic B w/Amp Mix, 50ml; Mic B-Complex 30ml; Mic B-Complex 50ml; Mic Combo w/methyl 30ml; Mic Ultra 10ml; Mic Ultra 30ml; Mic Ultra 50ml; Mic Ultra 50ml-Sulfur Free; Mic Ultra 50ml-Sulfur Free w/Lido; Mic Ultra Sulfur Free 10ml; Mic Ultra Sulfur Free 30ml; Mic w/B6/B12/ Lido 50ml; Minoxidil 10% 60ml; Minoxidil 15% 60ml; Mitomycin 0.02%, 2.5ml; Mitomycin 20mg; Mitomycin 2ml; Mitomycin 40mg; Mitomycin 40mg Compounded; Mitomycin C; Mitomycin/Mannitol; Oral B-12 30ml cotton candy; Phosphatidylcholine/Deoxycholic Acid 50ml; Poison Ivy Extract 10ml; Pontocaine 2% 4oz; Post-menopausal HCG pack; Post-menopausal HCG Pk 2nd mont; Post-menopausal HCG Pk 3rd mont; Pre-menopausal HCG pack; Pre-menopausal HCG PK 2nd month; Pre-Menopausal Pack 3rd Month; Sermorelin 15mg; Sermorelin 6mg; Sermorelin GHRP 9mg/3mg/3mg; Sermorelin 3mg; Single Strength PGE1 20mcg 2ml; Single Strength PGE 1 20mcg/ml; Sodium Hyaluronate. 2ml; Sodium Hyaluronate 6ml; Sodium Tetradecyl sulfate 2.0% 30ml; Sodium Tetradecyl Sulfate 3.0%, 30ml; Sodium Tetradecyl Sulfate 5.0% 30ml; Sodium Thiosulfate 25% 50ml; Special Mic Formula 30ml; Taper A prefilled syringes; Taper Pack B; Taper Pack C; TCA 20% 30ml vial; TCA 30% 0.8PH 15ml vial; TCA 30% 30ml vial; Test Cypionate 20mg/ml 5ml; Test Cypionate/Prop 195/1.25mg/ml 10m; Testosterone Cypionate/Propionate 250mg/ml 10ml vial; Testosterone Cypionate/Testosterone Propionate 250mg 10ml; Testosterone 200mg, 10ml; Testosterone 200mg, 30ml; Testosterone 210mg 10ml; Testosterone Cypionate, 200mg 10ml; Testosterone Cypionate, 250mg 10ml; Testosterone Cypionate w/lidocaine 10ml; Testosterone Cypionate 100mg/ml; Testosterone Cypionate 200mg/ml 10ml; Testosterone Cypionate 210mg 10ml; Testosterone Cypionate 300mg/ml 10ml; Testosterone Pellet 112.5mg; Testosterone Pellet 112mg; Testosterone Pellet 12.5mg; Testosterone Pellet 87.5mg; Testosterone Pellets 100mg; Testosterone Pellets 125mg; Testosterone Pellets 200mg; Testosterone Pellets 220mg; Testosterone Pellets 225mg; Testosterone Pellets 25mg; Testosterone Pellets 37.5mg; Testosterone Pellets 50mg; Testosterone Pellets 75mg; Testosterone Propionate 100mg 10ml; Tetracaine 2%; Tetracaine 2% Pontocaine, 60ml; Triamcinolone Diacetate 40mg 2ml PF; Triamcinolone Diacetate 40mg 5ml; Triamcinolone PF 40mg 2ml; Triamcinolone 40mg PF; Triamcinolone Diacetate 40mg 10ml; Triamcinolone 40mg PF 10ml; Triamcinolone Acetaonide 40mg/ml; Tri-Mix SS Pap 30mg Phen 1mg; Tri-Mix SS Pap 30mg Phen 1mg, PGE1 10mcg; Trypan Blue 0.15% 1ml; Wydase 1ml; and Wydase 5ml. |
|||
|
Badger Baby Broad Spectrum SPF 30 Zinc Oxide Sunscreen Lotion Chamomile & Calendula, 36,272 4fl oz tubes; and Badger Kids Broad Spectrum SPF 30 Zinc Oxide Sunscreen Lotion Tangerine & Vanilla, 5,941 4fl oz tubes |
W.S. Badger Co. Inc., Gilsum, NH |
II |
Microbial Contamination of Non-Sterile Products; Selected lots of Badger Baby and Kids Sunscreen Lotion were recalled due to microbial contamination. |
|
Morphine Sulfate Injection USP, 250mg, 10mL Fill, 29,700 single-dose flip top vials |
Hospira Inc., Lake Forest, IL |
II |
Lack of Assurance of Sterility: Confirmed customer report of leakage of vial contents due to the breaking of the vial neck. |
|
15 oral solids recalls *
|
Novartis Pharmaceuticals Corp., Suffern, NY |
II |
Chemical Contamination: Novartis Pharmaceuticals Corporation has recalled physician sample bottles of Diovan, Exforge, Exforge HCT, Lescol XL, Stalevo, Tekturna and Tekturna HCT Tablets due to contamination with Darocur 1173 a photocuring agent used in inks on shrink-wrap sleeves. |
|
* 4.7 million physician sample bottles: Diovan (valsartan) 160mg per tablet, 7 tablets per bottle, 139,788 bottles; Diovan (valsartan) 320mg per tablet, 7 tablets per bottle, 57,881 bottlers; Exforge (amlodipine and valsartan tablets) 5/160mg, 7 tablets per bottle, 1,492,475 bottles; Exforge (amlodipine and valsartan tablets) 5/320mg, 7/bottle, 869,951 bottles; Exforge HCT (amlodipine, valsartan and hydrochlorothyazide tablets) 10/320/25mg, 7/bottle, 281,935 bottles; Exforge HCT (amlodipine, valsartan and hydrochlorothyazide tablets) 5/160/12.5mg, 7/bottle, 328,543 bottles; Exforge HCT (amlodipine, valsartan and hydrochlorothyazide tablets) 5/160/25mg, 7/bottle, 174,848 bottles; Exforge (amlodipine and valsartan tablets) 10/160mg, 7/bottle, 61,240 bottles; Exforge (amlodipine and valsartan tablets) 10/360mg, 7/bottle, 951,109 bottles; Lescol XL (fluvastatin sodium) Extended-Release Tablets 80mg per tablet, 7 tablets per bottle, 46,812 bottles; Stalevo (carbidopa, levodopa and entacapone) 12.5/50/200mg tablets, 7/bottle, 18,665 bottles; Tekturna (aliskiren) Tablets 150mg, 7/bottle, 126,245 bottles; Tekturna (aliskiren) Tablets 300mg, 7/bottle, 128,964 bottles; Tekturna HCT (aliskiren and hydrochlorothiazide) Tablets 150/12.5mg, 7/bottle, 16,693 bottles; and Tekturna HCT (aliskiren and hydrochlorothiazide) Tablets 300/25mg, 7/bottle, 70,006 bottles. |
|||
|
10% Travasol (Amino Acid) Injection, 2000ml pharmacy bulk package, 14,622 bags |
Baxter Healthcare Corp., Deerfield, IL |
II |
Lack of Assurance of Sterility; Drug product leaking from container, therefore sterility cannot be assured. |
|
Carisoprodol Tablets, USP 350mg, 20,534 bottles, 1,000 tablets each |
West-ward Pharmaceutical Corp., Eatontown, NJ / Shasun Chemicals and Drugs Limited, Pondicherry, 605014, India |
II |
Presence of Foreign Substance; heavy metals (chromium, titanium etc) and inactive components of the product were visually observed during routine stability testing. |
|
Crest Pro-Health CPC Antigingivitis/ Antiplaque Oral Rinse, 250 mL/bottle, 71,040 bottles |
Procter & Gamble Hair Care, Iowa City, IA |
III |
Cross Contamination with other products: Product was mixed with another type of mouth wash. |
|
Montelukast Sodium Tablets, 10 mg, 30 or 90/bottle, 591,972 bottles |
Glenmark Generics Inc. Mahwah, NJ / Glenmark Generics Ltd., Colvale-Bardez, Goa, India |
III |
Chemical Contamination: The recall has been initiated based on multiple complaints received from pharmacists and consumers reporting that they detected an off-odor, described as moldy, musty or fishy in nature which has been identified as trace levels of Tribromoanisole (TBA) and Trichloroanisole (TCA). |
|
Torisel Kit (temsirolimus) injection, 25mg/mL, Concentrated, 10,920 kits, each with one vial of Torisel and one vial of diluent |
Pfizer Inc., New York, NY |
III |
Lack of Assurance of Sterility; potential that a low level of endotoxins may be present in the diluent vials. |
|
Femhrt tablets, 0.5 mg/2.5mcg, 90/bottle, 27,509 bottles; Jevantique tablets; 1.0mg/5.0mcg, 90/bottle, 17,136 bottles; Femhrt tablets, 1.0 mg/5.0mcg, 90/bottle, 6,408 bottles |
Warner Chilcott Company LLC., Fajardo, PR |
III |
Chemical contamination: Firm's inspection discovered the presence of 2-phenylphenol in the product due to migration from the cardboard cartons in which the product is packaged. |
|
Percocet 10/325 mg tablets, one bottle containing 100 tablets |
Physicians Total Care, Inc. Tulsa, OK / Novartis Consumer Health., Lincoln, NE |
III |
Presence of Foreign Tablets/Capsules: One bottle of Percocet 10/325 mg was found to contain a tablet of Endocet 10/25 mg, the generic form. |
|
Plavix 75 mg, two 30-count bottles |
Physicians Total Care, Inc. Tulsa, OK / Bristol-Myers Squibb, Bridgewater, NJ |
III |
Chemical Contamination: Uncharacteristic moldy odor due to presence of 2,4,6 tribromoanisole. |
|
Pharmalucence Kit for the Preparation of Technetium Tc 99m Sestamibi injection for myocardial and breast imaging, 17,260 vials in five- and 30-vial kits |
Pharmalucence, Inc., Billerica, MA |
III |
Presence of Particulates; particulate found in retain sample. |
|
ALCOHOL Free Antiseptic (cetylpyridinium chloride) Mouth Rinse, 0.07% mint, 18,408 bottles |
Vi-Jon, Inc., Smyrna, TN |
III |
Microbial Contamination of Non-Sterile Products: This product is being recalled because a stability sample was found to be contaminated with Burkholderia contaminans. |
|
TUMS, Antacid/Calcium Supplement, ULTRA Strength 1000 Assorted Berries, 72 chewable tablets per bottle, 300,480 bottles |
GlaxoSmithKline, LLC, Zebulon, NC / GlaxoSmithKline, Moon Township, PA |
III |
Presence of Foreign Tablets/Capsules: Product labeled TUMS Ultra Assorted Berries 1000mg Chewable tables, may contain EX TUMS assorted berries 750mg tablets. |
|
SPIRIVA/HandiHaler (tiotropium bromide inhalation powder) Capsules 18mcg per dose, 15,385,232 capsules |
Boehringer Ingelheim Roxane Inc., Columbus, OH / Boehringer Ingelheim , Ingelheim, Germany |
III |
Presence of Foreign Substance: This recall is being conducted due to the potential for extrinsic foreign particles in the API used to manufacture SPIRIVA handihaler. |
|
Manufacturing / Testing Methods |
|||
|
Product(s)
|
Recaller / Manufacturer |
Class
|
Reason
|
|
Warfarin Sodium Tablets, USP 2mg 1000 Tablets per bottle, 960 bottles |
Zydus Pharmaceuticals USA, Pennington, NJ / Cadila Healthcare Ltd., Ahmedabad, India |
I |
Failed Tablet/Capsule Specifications: A product complaint was received from a pharmacist who discovered that 3 tablets in a 1000-count bottle were oversized. |
|
Medi-bolic Booster Injectable (methionine, choline chloride, cyanocobalamin, chromium chloride 126 multi-dose vials, 30 mL each |
Rx South DBA Rx3 Pharmacy, Chester, VA |
II |
CGMP Deviations: Pharmaceutical for injection was not manufactured according to Good Manufacturing Procedures. |
|
AMBI cough/cold oral liquids, various combinations of antitussive, expectorant, nasal decongestant and cough suppressant, various flavors and amounts, 4,541 bottles |
AMBI Pharmaceuticals, Brooksville, FL / TG United, Inc., Brooksville, FL |
II |
CGMP Deviations: Products are underdosed or have an incorrect dosage regime. |
|
AMBI 40PSE/400GFN/20DM, Cough Suppressant, Expectorant, Nasal Decongestant, 1,772 100-count bottles; AMBI 40PSE/400GFN, 886 100-tablet bottles |
AMBI Pharmaceuticals, Brooksville, FL / TG United, Inc., Brooksville, FL |
II |
CGMP Deviations: Products are underdosed or have an incorrect dosage regime. |
|
Ryddex G Tablets, Decongestant, Expectorant, 100 count bottle, 2,957 |
Centurion Labs, LLC, Richland, MS / TG United, Inc., Brooksville, FL |
II |
CGMP Deviations: Products are underdosed or have an incorrect dosage regime. |
|
ED A-HIST Tablets, Antihistamine, Nasal Decongestant, 242,470 100 count bottles |
Edwards Pharmaceuticals Inc., Ripley, MS / TG United, Inc., Brooksville, FL |
II |
CGMP Deviations: Products are underdosed or have an incorrect dosage regime. |
|
NoHist Tablets, Antihistamine, Nasal Decongestant, 100 count bottle, 4,824 |
Larken Laboratories, Inc., Canton, MS / TG United, Inc., Brooksville, FL |
II |
CGMP Deviations: Products are underdosed or have an incorrect dosage regime. |
|
Dallergy TABLETS, Antihistamine, Nasal Decongestant, 100 count bottle , 31,739; Dallergy Chewable Tablets, Antistamine, Nasal Decongestant, 100 count bottle , 8,296 |
Laser Pharmaceuticals, Greenville, SC / TG United, Inc., Brooksville, FL |
II |
CGMP Deviations: Products are underdosed or have an incorrect dosage regime. |
|
Cardec Drops, Antihistamine, Nasal Decongestant, 216,838 bottles with Antitussive, 26,536 without, 1 fl oz. bottle |
Macoven Pharmaceuticals, Magnolia, TX / TG United, Inc., Brooksville, FL |
II |
CGMP Deviations: Products are underdosed or have an incorrect dosage regime. |
|
Brompheniramine/Pseudophedrine DM, Liquid, Antihistamine, Cough Suppressant, Decongestant, 22,721 16-oz bottles |
Macoven Pharmaceuticals, Magnolia, TX / TG United, Inc., Brooksville, FL |
II |
CGMP Deviations: Products are underdosed or have an incorrect dosage regime. |
|
Maxifed DM, Expectorant, Nasal Decongestant, 100 count bottle 2,706; Maxifed-G, Expectorant, Nasal Decongestant, 100 count bottle , 1,623 |
MCR American Pharmaceuticals, Brooksville, FL / TG United, Inc., Brooksville, FL |
II |
CGMP Deviations: Products are underdosed or have an incorrect dosage regime. |
|
BroveX PSB Liquid, Antihistamine, Decongestant, 65,412 16-oz bottles; BroveX PSB DM Liquid, Antihistamine, Decongestant, 39,744 16-oz bottles; Lusair Liquid, 1 PINT bottle |
Pernix Therapeutics, Gonzales, LA / TG United, Inc., Brooksville, FL |
II |
CGMP Deviations: Products are underdosed or have an incorrect dosage regime. |
|
BroveX PSE, Antihistamine, Nasal Decongestant, 100 count bottle , 10,036; BroveX PSE, Antihistamine, Cough Suppressant, Decongestant, 100 count bottle, 16,836 |
Pernix Therapeutics, Gonzales, LA / TG United, Inc., Brooksville, FL |
II |
CGMP Deviations: Products are underdosed or have an incorrect dosage regime. |
|
Poly Hist Forte, Nasal Decongestant, Antihistamine, 100 count bottle, 333,158; Poly Vent DM Tablets, Cough Suppressant, Expectorant, Nasal Decongestant, 60 count bottle, 977,719; Poly Vent IR, Poly-Vent DM Tablets, Expectorant, Nasal Decongestant, 60 count bottle, 978,092 |
Poly Pharmecuticals, Quitman, MS / TG United, Inc., Brooksville, FL |
II |
CGMP Deviations: Products are underdosed or have an incorrect dosage regime. |
|
TG 40PSE/400GFN Tablets, 1000 count bottle , 386 |
TG United, Inc., Brooksville, FL / TG United, Inc., Brooksville, FL |
II |
CGMP Deviations: Products are underdosed or have an incorrect dosage regime. |
|
6 nasal product recalls *
|
Trigen Laboratories, Sayreville, NJ / TG United, Inc., Brooksville, FL |
II |
CGMP Deviations: Products are underdosed or have an incorrect dosage regime. |
|
* CPM/PSE Drops, Antihistamine, Nasal Decongestant, 6,6551 1 fl oz bottle; CPM/PSE DM Drops, Antihistamine, Cough Suppressant Decongestant, 26,315 1fl oz bottle; TL Hist PD Drops, Antihistamine, Nasal Decongestant, 13,031 1 fl.oz bottle; Trigofen Drops, Antihistamine, Decongestant, 39,403 1 fl oz bottle; Trigofen DM Drops, Antihistamine, Cough Suppressant, Decongestant, 40,149 1 fl oz bottle; ZoDen DM Drops, Antihistamine, Cough Suppressant, Decongestant, 39,801 1 fl oz. bottle |
|||
|
8 oral liquids recalls *
|
Trigen Laboratories, Sayreville, NJ / TG United, Inc., Brooksville, FL |
II |
CGMP Deviations: Products are underdosed or have an incorrect dosage regime. |
|
* Mesehist DM, Antihistamine, Antitussive, Decongestant, 12,120 16 fl oz bottle; Mesehist WC, Antihistamine, Antitussive, Decongestant, 4,884 16fl oz bottle ; TL-DEX DM, Cough Suppressant Decongestant, Expectorant, 2,532 16fl oz. bottle ; TL Hist DM, Antihistamine, Decongestant, Cough Suppressant, 98,202 16fl oz. bottle ; Tri-Dex PE, Antihistamine, Antitussive, Nasal Decongestant, 12,198 16 fl oz bottle ; Z-Dex Pediatric Drops, Cough Suppressant Decongestant, Expectorant, 12,988 16 fl oz bottle; Z-Dex Syrup, Decongestant, Antitussive, Expectorant, 2,388 16fl oz bottle; ZoDen PD, Antihistamine, Nasal Decongestant, 4,811 16 fl oz bottle |
|||
|
Infants Gripe Mixture, Each 5ml contains: Light Magnesium Carbonate BP, 200 mg Sodium Bicarbonate BP, 100 mg; Sucrose 487.5mg; Alcohol 0.362 mL; Fennel Oil BP 0.004 mL; Dill Oil BP, 0.005 mL, 120 mL bottle, 480 bottles |
Carib Import & Export Inc., Miami, FL / P.A. Benjamin Manufacturing Co, Kingston, Jamaica |
II |
CGMP Deviations: this product is being recalled because an FDA inspection revealed that it was not manufactured under current good manufacturing practices. |
|
Pantoprazole Sodium Delayed Release Tablets USP, 40mg, 12,770 90-count bottles |
Jubilant Cadista Pharmaceuticals Inc., Salisbury, MD / Jubilant Life Sciences Ltd, Roorkee, India |
II |
cGMP Deviations: Oral products were not manufactured in accordance with Good Manufacturing Practices. |
|
Fludeoxyglucose F 18 Injection USP, 20-200mCi/mL , 28 doses |
Petnet Solution Inc., Irvine, CA / PETNET Solutions, Inc., Knoxville, TN |
II |
cGMP Deviation |
|
Cefdinir for Oral Suspension, 125 mg/5 mL 60 mL bottle and 100 mL bottle, 6,2187 bottles |
Teva Pharmaceuticals USA, Inc., Sellersville, PA |
II |
Defective Container: This recall is being carried out due to the potential for improperly sealed bottles. |
|
Venlafaxine Hydrochloride Tablets, 75mg, 100 count tablets per bottle, 13,320 bottles |
Zydus Pharmaceuticals USA, Pennington, NJ / Cadila Healthcare Ltd., Ahmedabad, India |
II |
Failed Tablet/Capsule Specifications: Pharmacist complaint of an excessive amount of broken and/or chipped tablets in the bottle. |
|
20 oral solids recalls *
|
Lloyd Inc. of Iowa, Shenandoah, Iowa / Some were manufactured for Forest Pharmaceuticals Inc., St. Louis, MO |
II |
cGMP deviations; After quality review of stability failures in previous lots, there is insufficient data to determine that other lots are not affected. |
|
* 4,440 100-tablet bottles of Levothroid (levothyroxine sodium tablets, USP), 50 mcg; 7,681,277 Lloyd Thyro-Tab 0.050 mg. tablets packaged in 150,000-tablet bulk drums; 19,787 bottles of Levothroid (levothyroxine sodium tablets, USP), 75 mcg; 9,545,718 Lloyd Thyro-Tab 0.075 mg. tablets packaged in 150,000-tablet bulk drums; 12,156 bottles of Levothroid (levothyroxine sodium tablets, USP), 88 mcg; 5,767,711 Lloyd Thyro-Tab 0.088 mg. tablets, packaged in 150,000-tablet bulk drums; 21,019,909 Lloyd Thyro-Tab 0.100 mg. tablets, packaged in 150,000-tablet bulk drums for repackaging; 7,614,709 Lloyd Thyro-Tab 0.112 mg. tablets, packaged in 150,000-tablet bulk drums for repackaging; 13,460,920 Lloyd Thyro-Tab 0.125 mg. tablets, packaged in 150,000-tablet bulk drums for repackaging; 3,844,402 Lloyd Thyro-Tab 0.137 mg. tablets, packaged in 150,000-tablet bulk drums for repackaging; 9,575,108 Lloyd Thyro-Tab 0.150 mg. tablets, packaged in 150,000-tablet bulk drums for repackaging; 5,768,782 Lloyd Thyro-Tab 0.175 mg. tablets, packaged in 150,000-tablet bulk drums for repackaging; 5,771,989 Lloyd Thyro-Tab 0.200 mg. tablets, packaged in 150,000-tablet bulk drums for repackaging; 142,103 100-tablet bottles of Levothroid (levothyroxine sodium tablets, USP), 100 mcg; 40,311 bottles of Levothroid (levothyroxine sodium tablets, USP), 112 mcg; 77,471 bottles of Levothroid (levothyroxine sodium tablets, USP), 125 mcg; 12,911 bottles of Levothroid (levothyroxine sodium tablets, USP), 137 mcg; 61,347 bottles of Levothroid (levothyroxine sodium tablets, USP), 150 mcg; 32,345 bottles of Levothroid (levothyroxine sodium tablets, USP), 175 mcg; and 29,489 bottles of Levothroid (levothyroxine sodium tablets, USP), 200 mcg. |
|||
|
3M Tekk Brand Industrial/Construction First Aid Kit; Medi-First Brand First Aid Kit, MEDI-First 61 piece kit; and First Aid Kit, 326 Piece Kit |
Total Resources Intl, City of Industry, CA |
II |
CGMP Deviations: The first aid kits that are subject to the voluntary recall is being initiated due to failure in stability testing of the active ingredient Benzalkonium Chloride. |
|
CLENZIderm M.D. Acne Therapeutic System Normal to Oily Skin kit, salicylic acid 2%, benzoyl peroxide, 5%, 2,491 kits |
Medicis Pharmaceutical Corp., Scottsdale, AZ |
II |
cGMP Deviations; during the production process, CLENZiderm M.D. Daily Foam Cleanser was filled into some bottles labeled as CLENZiderm M.D. Pore Therapy. |
|
4 oral solids recalls *
|
Novartis Consumer Health, Lincoln, NE |
II |
Defective container: products are packaged in pouches which may not have been fully sealed. |
|
* Excedrin Extra Strength, 250 mg acetaminophen, 250 mg aspirin and 65 mg caffeine tablets in 4,178,000 two-count pouches, 101,300 boxes of 50 two-count pouches, and 38,235 boxes of 48 four-count pouches for U.S., Puerto Rico, Brazil and Panama; Excedrin Migraine, 250 mg acetaminophen, 250 mg aspirin, 65 mf caffeine, in 10,624,000 two-count pouches; No Doz, Max Strength caplets, 200 mg caffeine, 4,422,000 two-count pouches; Parsel Plus (Excedrin Tension Headache in the U.S.), 500 mg acetaminophen, 65 mg caffeine, 472,000 two-count pouches, distributed to Novartis Brazil |
|||
|
Vecuronium Bromide for Injection, 10mg, 831,950 vials |
Sagent Pharmaceuticals, Schaumburg, IL / MN Pharmaceuticals, Turkey |
II |
CGMP Deviations: product was not manufactured under current good manufacturing practices which contributed to Failed Impurities/Degradation Specifications as a high out of specification impurity result was detected during routine quality testing of stability samples. |
|
Olanzapine Tablets, USP, 10mg, 30/bottle, 18,721 bottles |
Torrent Pharma Inc., Kalamazoo, MI / Torrent Pharmaceuticals Ltd, Mehsana India, for Prasco Laboratories, Mason, OH |
II |
Defective Container; This action is being taken as a precautionary measure due to the product being re-packaged in the U.S. using filler material that (removes or blocks) less moisture than what is approved in the application. |
|
Dianeal Low Calcium Peritoneal Dialysis Solution with 1.5% Dextrose, 39,486 5000mL containers |
Baxter Healthcare Corp., Deerfield, IL |
II |
Defective Container: There is a potential for frangible components to be broken, resulting in a leak at the port when the closure is removed. |
|
Acetaminophen Tablets, 500mg, 28,387 100-count bottles |
Marlex Pharmaceuticals, New Castle, DE |
II |
CGMP Deviations: The recalled acetaminophen tablet lot was not manufactured under current good manufacturing practices as noted by a recent inspection of the manufacturing firm. |
|
Ifosfamide Injection, 49 1g/20mL single-dose vials; 60 3g/60mL single-dose vials |
AmeriSource Bergen, Chesterbrook, PA |
II |
Temperature abuse: Certain vials of Ifosfamide IV products were not refrigerated at certain Amerisource Bergen Drug Corp distribution centers. |
|
12 oral solids recalls *
|
Mylan Pharmaceuticals Inc., Morgantown, WV |
II |
CGMP Deviations: Pharmaceuticals were produced and distributed with active ingredients not manufactured according to Good Manufacturing Practices. |
|
* AmIodipine Besylate Tablets, USP 2.5mg, 37,752 90-count and 500-count bottles; AmIodipine Besylate and Benazepril HCI Capsules, 2.5mg/10mg, 4,722 100-count bottles; Lamotrigine Tablets, USP 200mg, 28,140 60-count and 500-count bottles; Ciprofloxacin Tablets, USP 250mg, 521 100-count bottles; AmIodipine Besylate Tablets, USP 5mg, 167,866 90-count and 500-count bottles; AmIodipine Besylate and Benazepril HCI Capsules, 5mg/10mg, 30,878 100-count and 500-count bottles; AmIodipine Besylate and Benazepril HCI Capsules, 5mg/20mg, 60,506 100-count and 500-count bottles; AmIodipine Besylate and Benazepril HCI Capsules, 5mg/40mg, 11,701 100-count bottles; AmIodipine Besylate and Benazepril HCI Capsules, 10mg/20mg in 42,174 100-count and 500-count bottles; AmIodipine Besylate and Benazepril HCI Capsules, 10mg/40mg, 23,356 100-count bottles; Ciprofloxacin Tablets, USP 500mg, 941,270 100-count bottles; and AmIodipine Besylate Tablets, USP 10mg, 101,450 90-count and 500-count bottles. |
|||
|
Lactated Ringer's Injection, USP 1000 mL, 1,296 units |
Hospira, Inc., Lake Forest, IL |
III |
CGMP Deviations: Shipment of product not approved for release. |
|
EstroGel (estradiol gel) 0.06%, contained in 50-gram metered pump bottles, 1.25 grams/pump depression, for physician starter samples, 4,835 bottles |
Ascend Therapeutics Inc., Herndon, VA / DPT Laboratories, San Antonio, TX |
III |
Defective Container: Pump head detaching from the canister unit upon removal of the overcap. |
|
Flanax, Fast Acting Antacid, 200mg, 12fl oz bottles, 17,996 bottles |
Belmora LLC, Arlington, VA |
III |
Defective Container: Product lacks tamper evident breakaway band on cap. |
|
Disulfiram Tablets USP 250mg, 100 tablet bottle, 68,322 bottles |
Teva Pharmaceuticals USA, Sellersville, PA |
III |
CGMP Deviation; cotton coil is missing in some packaged bottles |
|
10,362 Braintree HalfLytely and Bisacodyl Tablet Bowel Prep Kits |
Braintree Laboratories Inc., Holbrook, MA |
III |
Defective Container; Small micro fracture observed in the 2-Liter bottle at the fill line resulting in a small leak when patient reconstitutes the bulk powder. |
|
Labeling / packaging mix-ups |
|||
|
Product(s)
|
Recaller / Manufacturer |
Class
|
Reason
|
|
Rugby Natural Iron Supplement Ferrous Sulfate, 5gr, 19,944 100-tablet bottles |
Rugby Laboratories, Duluth, GA / Advance Pharmaceutical Inc., Holtsville, NY |
I |
Labeling; Label Mixup; bottles of Ferrous Sulfate actually contains Meclizine HCI (indicated for motion sickness) |
|
ENTERIC Coated Aspirin, 81mg, 16,440 120-count bottles |
Rugby Laboratories Inc., Duluth, GA / Advance Pharmaceutical Inc., Holtsville, NY |
I |
Labeling: Label Mix-Up: A complaint from a pharmacist was received that the entire contents of 1 bottle labeled as Rugby label Enteric Coated Aspirin 81 mg Tablets actually contained Acetaminophen 500 mg Tablets. |
|
Eye Drops advanced relief (Dextran 70. 0.1%, Polyethylene glycol 400 1%, Povidone 1%, Tetrahydrozoline HCI 0.05%) ophthalmic solution, 31,704 bottles, 15 mL each |
KC Pharmaceuticals Inc., Pomona, CA |
II |
Labeling: Label Mix-Up: Incorrect back labeling of the immediate container of eye drops (unit carton and front label are correct) which do not list three active ingredients: Dextran 70, PEG 400, and povidone. |
|
Synthroid (Levothyroxine Sodium) tablets, 150 mcg (0.15 mg), 90 tablets per bottle, 28,524 bottles |
Abbott Laboratories, North Chicago, Ill. / Abbott Laboratories, Abbott Park, Ill. |
II |
Labeling: Error on Declared Strength. Product labeled to contain 150 mcg tablets actually contained 75 mcg tablets. |
|
Donnatal Extentabs, 0.3888mg/46.8mg, 1,258 100-count bottles |
West-ward Pharmaceutical Corp., Eatontown, NJ / West-ward Pharmaceutical Corp., Eatontown, NJ, for PBM Pharmaceuticals, Inc., Gordonsville, VA |
II |
Labeling: Incorrect or Missing Lot and/or Exp Date: Bottled product is labeled with an expiration date of Apr 2015. The correct expiration is Apr 2013. |
|
0.9% Sodium Chloride Injection, USP 50 mL Mini-Bag Plus Container, 317,280 bags |
Baxter Healthcare Corp., Deerfield, IL |
II |
Labeling Wrong Barcode; It may display wrong product code reflecting 0.9% Sodium Chloride Injection, USP 100 mL in MINI-BAG Plus container instead of 50 mL. |
|
Voltaren 1% Gel, 76 100-gm tubes |
Physicians Total Care, Inc. Tulsa, OK / Novartis Consumer Health, Parsippany, NJ |
II |
Labeling: Incorrect or Missing Lot and/or Expiration Date; This recall is being initiated because the lot number and expiration date on the tube may not be legible. |
|
SEPP 70% Isopropanol, USP, 698.000 applicators, 200/carton; SEPP 2% Iodine Tincture USP, 3,032,000 applicators, 200/carton; SEPP 10% Povidone Iodine Solution USP, 8,960,000 applicators, 200/carton and 3,000/case; blood culture prep kit (70% isopropyl alcohol and 2% iodine tincture), 929,000 kits; donor prep kits (70% isopropyl alcohol and 2% iodine tincture), 46,500 kits; and 544,500 FREPP/SEPP kits (povidone iodine 2% and 10%), 20/carton. |
CareFusion 213, LLC, El Paso, TX / CareFusion, Leawood, KS |
II |
Labeling: Not Elsewhere Classified: This product is misbranded because the product is not sterile and the labeling is misleading in relation to sterility claims. |
|
Children's Acetaminophen Oral Suspension, 650 mg/20.3 mL unit dose cup, 30 cups/case, 9,268 cases |
Precision Dose, Inc., South Beloit, IL |
II |
Labeling: Not Elsewher Classified: This unit dose product is being recalled because the product's name includes the word "Children's" which is misleading since the 650 mg dose of acetaminophen contained within it, is an adult dose. |
|
Pentrexcilina (acetaminophen, chlorpheniramine maleate, phenylephrine HCl) tablets, 20 per carton; Pentrexcilina Jarabe NF (dextromethorphan HBr and guaifenesin) liquid, 118 mL/4 fl oz bottle |
OPMX, LLC, San Diego, CA |
II |
Labeling: Not Elsewhere Classified: Pentrexcilina Tablets and Pentrexcilina Liquid are being recalled because the product labels are misleading because they may be confused with Pentrexyl, a prescription antibiotic used to treat respiratory illnesses in Mexico. |
|
Walmart Amlodipine Besylate Tablets, USP 10 mg, 30/carton, 484,236 cartons |
Camber Pharmaceuticals, Inc., Piscataway, NJ / InvaGen Pharmaceuticals Inc., Hauppauge, N.Y. |
II |
Labeling: Labeling Error on Declared Strength; product label incorrectly states Amlodipine 5mg instead of Amlodipine 10mg. Tablet strength is 10mg. |
|
3,048 ropinirole hydrochloride tablets, USP 4 mg, 100-count bottles |
Mylan Pharmaceuticals Inc., Morgantown, WV |
II |
Labeling: Label Error on Declared Strength: Unopened bottles of Ropinirole USP 3mg tablets was found to be incorrectly labeled as Ropinirole USP 4mg tablets. |
|
Pure-Aid Allergy Relief, Diphenhydramine HCL Caplets, 25mg, 24,048 cartons |
Kareway Product Inc., Compton, CA |
II |
Labeling: Not Elsewhere Classified; foil label on immediate blister pack indicates active ingredient as Chlorpheniramine rather than Diphenhydramine. |
|
Zolpidem Tartrate Tablets 5mg, 10 tablets per blister, 10 blisters per carton, 100 cartons per box, 48,230 carton units |
American Health Packaging, Columbus, OH / Wockhardt Limited, Mumbai, India |
II |
Unit Dose Mispackaging: This recall event is due to a random undetected packaging issue which could increase the potential for a small number of individual unit dose blisters to be packed with more than one tablet. |
|
Estarylla (norgestimate and ethinyl estradiol) Tablets, USP, 0.25 mg/0.035 mg, 3X28/carton, 10,848 cartons |
Sandoz Inc., Broomfield, CO / Laboratorios Leon Farma S.A, Spain for Sandoz Inc., Princeton, NJ |
II |
Contraceptive Tablets out of Sequence: A placebo tablet was found in a row of active tablets. |
|
DayQuil Sinex DayTime Sinus Relief and NyQuil Sinex NightTime Sinus Relief, acetaminophen 325 mg, phenylephrine HCl 5 mg, doxylamine succinate 6.25 mg, 48/carton, 35,568 cartons |
Procter & Gamble Co., Mason, OH / Made in Canada |
II |
Unit Dose Mispackaging: Product packaging defect which could result in code date smearing, inccomplete blister card cuts, and missing or incorrectly placed liquicaps within the blisters. |
|
K Effervescent Tablets, Potassium (977 mg), Potassium Bicarbonate Effervescent Tablets for oral solution, 1,680 boxes; Effervescent Potassium/Chloride Tablets, Potassium Chloride (1,865 mg), 30-count tablets for oral solution, 1,680 boxes. |
Qualitest Pharmaceuticals, Huntsville, AL / Tower Laboratories Ltd., Centerbrook, CT |
II |
Labeling: Label Mix-up: 30 count Effervescent Potassium/Chloride Tablets, may be labeled as 30 count K Effervescent Tablets. |
|
IBU (TM) Ibuprofen tablets, USP, 800 mg, 18,852 500-count bottles |
Dr. Reddys Laboratories, Bridgewater, NJ |
II |
Labeling: Incorrect or missing lot and/or exp date- This product is being recalled due to an incorrect expiration date of 05/2017. The correct expiration date is 10/2016. |
|
Vicks NyQuil Cold and Flu Nighttime Relief, 11,337 Liquid Twin Packs, (Acetaminophen 650mg, Dextromethorphan HBr 30mg, Doxylamine succinate 12.5mg), 12 fl oz (354 ml) bottles |
Procter & Gamble Co., Mason, OH |
II |
Incorrect/ Undeclared Excipients: NyQuil Liquid Original bottles were inadvertently overwrapped with NyQuil Liquid Cherry information as a result the outer wrap does not correctly identify color additives, particularly FD&C Yellow No. 6 and FD&C Yellow 10. |
|
Acetaminophen suspension liquid, 160 mg/5mL, 2 fl oz bottles with an oral dosing syringe in a carton sold under various store brands and various flavors including: dye-free cherry flavor, 143,892 bottles; cherry flavor, 35,136 bottles; dye-free grape flavor, 27,504 bottles; and grape flavor, 108,828 bottles. |
L Perrigo Co., Allegan, MI |
II |
Defective Delivery System: There is a remote potential that cartons of product could be co-packaged with an oral dosing syringe without dose markings. |
|
CarisoprodoltTablets, USP, 350mg, 100/bottle, 1,763 bottles |
West-ward Pharmaceutical Corp., Eatontown, NJ |
III |
Labeling: Not elsewhere classified: On 12/12/11, DEA published a final rule in the Federal Register making this product a schedule IV (C-IV)controlled substance. This product is being recalled because this controlled product was not relabeled with the required "C-IV" imprint on the label for products distributed after the 06/11/12 deadline. |
|
Bronkaid Dual Action Formula, Ephedrine Sulfate 25 mg/ Bronchodilator, Guaifenesin 400mg/Expectorant, 1,353,420 blister packs of 24 or 60 coated caplets each |
Bayer HealthCare LLC, Consumer Care, Morristown,NJ |
III |
Labeling: Label lacks warning or Rx legend; Certain information was inadvertently excluded from the product carton label. |
|
Divalproex Sodium Delayed Release Tablets USP 500mg Valproic Acid Activity, 100 count tablets, 11,316 bottles |
Upsher Smith Laboratories, Inc., Maple Grove, MN / Upsher-Smith Laboratories, Inc., Minneapolis, MN |
III |
Labeling: Label Error On Declared Strength; Some bottles of product were missing the color-coded strength on the primary display panel of the label. |
|
Syeda (drospirenone and ethinyl estradiol), 3mg/0.03mg, 3x28 tablet blister cards, combination oral contraceptive tablet, 17,188 cartons |
Sandoz Inc., Broomfield, CO / Laboratorios Leon Farma S.A., Spain |
III |
Tablet/Capsule Imprinted with Wrong ID; a portion of the tablets could contain the incorrect debossing on one side of the tablet U2 instead of U3. |
|
Methocarbamol 750 mg Tablets, 90 count bottle, three bottles |
Stat Rx USA, Gainesville, GA / West-Ward Pharmaceuticals Corp., Eatontown, NJ |
III |
Labeling: Incorrect or missing lot and/or expiration date. The product was mistakenly labeled with an expiration date of 10/16 instead of 01/16 |
|
Calcium Gluconate Injection USP, 10% 10ml Single Dose Vial, 155,900 vials |
Fresenius Kabi USA, Schaumburg, IL / APP Pharmaceuticals Schaumburg, IL |
III |
Labeling: Missing label |
|
TEV-Tropin (somatropin rDNA origin for injection 5mg, 126 injectors |
Ferring Pharmaceuticals Inc., Parsippany, NJ / Manufactured in Israel |
III |
Labeling: Label Mix-Up: Units of one lot are packaged in cartons labelled for Needle-Free Head B, but contain Needle Free head A. |
|
Novartis Lamisil AT Cream for Jock Itch terbinafine hydrochloride , 219,600 tubes |
Novartis Consumer Health., Lincoln, NE / Novartis Consumer Health, Parsippany, NJ |
III |
The products have illegible lot and/or expiration dates on the product container. |
|
Novartis Lamisil AT Cream, Terbinafine Hydrochloride Cream 1% Antifungal, 1,654,440 tubes; Target Up & Up, athlete's foot cream, full prescription strength, terbinafine hydrochloride 1% antifungal 30g, 99,660 tubes; Lamisil AT Cream (terbinafine hydrochloride 1% antifungal) 12gm and 30gm tubes, 41,400 boxes |
Novartis Consumer Health., Lincoln, NE / Novartis Consumer Health, Parsippany, NJ |
III |
Labeling: Incorrect or Missing Lot and/or Expiration date; The Lot and/or Expiration date on the tube may not be legible in this lot. |
|
Voltaren Gel (diclofenac sodium topical gel) 1% for topical use only, 4,429,407 tubes |
Novartis Consumer Health., Lincoln, NE / Novartis Consumer Health, Parsippany, NJ |
III |
Labeling: Incorrect or Missing Lot and/or Expiraton date; The Lot and/or Expiration date on the tube may not be legible in this lot. |
|
Lisinopril Tablets, USP 10mg, 2,405 1000-count bottles |
Mylan Pharmaceuticals Inc., Morgantown, WV / Manufactured in India |
III |
Tablets/Capsules Imprinted With Wrong ID: Pharmaceuticals are imprinted with an incorrect identifier code on the embossed tablets. |
|
Myoflex (trolamine salicylate) 10% pain relieving cream, 240,120 2oz tubes and 429,144 4oz tubes; Nupercainal, Dibucaine, hemorrhoidal & topical analgesic ointment, 91,872 1oz tubes and 94,464 2oz tubes |
Novartis Consumer Health., Lincoln, NE |
III |
Labeling: Incorrect or Missing lot and/or expiration date.The lot number and/or expiration date may be illegible. |
|
Fast-acting nasal spray, phenylephrine HCl, 1%, 538,092 0.5oz bottles, 1,186,740 1oz bottles and 138,528 1.25oz bottles; menthol nasal spray with 1% phenylephrine HCl, 44,604 0.5fl oz bottles and 439,452 1fl oz bottles; and saline moisturizing mist with eucalyptol and menthol, 227,124 1fl oz bottles |
Novartis Consumer Health., Lincoln, NE |
III |
Labeling: Incorrect or Missing lot and/or expiration date.The lot number and/or expiration date may be illegible. |
|
Tizanidine Tablets USP, 4mg, 150-count tablets per bottle, 117,546 bottles |
Dr. Reddy's Laboratories, Inc., Bridgewater, NJ / Dr. Reddys Laboratories, Ltd., Bachepally, India |
III |
Labeling Illegible: There is a possibility that the bottle labels do not contain the strength of the product as well as other printing details. |
|
Lorazepam, 0.5mg tablets, 100 count, 1,644 bottles |
Legacy Pharmaceutical Packaging LLC, Earth City, MO / Major Pharmaceuticals, Livonia, MI |
III |
Labeling: Incorrect or Missing Lot and/or Exp Date |
|
Lisinopril tablets, USP, 30 mg, 1,776 bottles |
Aurobindo Pharma USA, Plainsboro, NJ / Aurobindo Pharma Ltd. Hyderabad, India |
III |
Labeling: Label Error on Declared Strength: Incorrect strength on side display panel of label. |
|
Lamisil AT (terbinafine hydrochloride cream 1% antifungal), 3,828 24 gram (0.85 oz) tubes |
Novartis Consumer Health., Lincoln, NE |
III |
Labeling: Incorrect or Missing Lot and/or Expiration date; The Lot and/or Expiration date on the tube may not be legible in this lot. |
|
Prednisone Tablet USP, 20mg tablets, 100/carton, 14,619 cartons |
Boehringer Ingelheim Roxane Inc., Columbus, OH |
III |
Labeling: Missing label; missing label on blister card. |
|
18 topicals recalls *
|
Tween Brands Inc., New Albany, OH |
III |
Labeling: Label Error on Declared Strength: the label states that the product contains 62% ethyl alcohol, but the ethyl alcohol content is 20%. |
|
* Ethyl alcohol, 590,435 1fl oz bottles: Believe in Yourself Strawberry Anti bac Justice; Share a Smile Mixed berry Anti bac Justice; Shine from within blueberry Anti bac Justice; Girls can change the World, Green Apple Anti bac Justice; Follow Your Dreams Justice, Chocolate anti bac; Today is Awesome Justice, Cotton Candy, anti back; Life is Sweet Justice, Vanilla Scent anti bac; I love my BFF Justice, Watermelon anti bac; I love sleep overs Justice, Grape anti bac; I love to shop Justice, Peach anti bac; Lemon anti bac Justice; Grapefruit anti bac Justice; GIRLS Rock Justice, Vanilla scent anti bac; I Mustache You to be my BFF Justice, Cotton Candy scent anti bac; I Love Bacon Justice, Vanilla scent anti bac; You Cant Handle This Justice, Grape Scent Anti Bac; yum yum bubble gum Justice, Bubblegum Scent anti bac; BFF's are COOL! Justice, Cherry Scent Anti Bac |
|||
|
Clobetasol Propionate Cream, USP, 0.05%, 15 grams, 58,812 tubes |
Hi-Tech Pharmacal Co., Inc, Amityville, NY |
III |
Labeling: Label Mix-up; some cartons labeled as Clobetasol Propionate Cream USP contain tubes labeled as Clobetasol Propionate Gel USP. The actual product in the tube is Clobestasol Propionate Cream USP. |
|
Mavik trandolapril tablets 4mg, 100 count bottle, 704 bottles |
AbbVie Inc., North Chicago, IL / Halo Pharmaceutical Inc., Whippany, NJ |
III |
Labeling; Incorrect Package Insert; product packaged with outdated version of the insert. |
|
9 oral solids recalls *
|
Novartis Consumer Health., Lincoln, NE / Novartis Consumer Health, Mississauga, ON |
III |
Labeling: Incorrect or Missing Lot and/or Exp Date: The lot number and/or expiration date may be illegible on the outer plastic bottle packaging. |
|
* Maalox chewable tablets (calcium carbonate sometimes with simethicone or alginic acid), various strengths, configurations, flavors and amounts: in 117,816 65-count bottles; in 112,728 50- 100- and 145-count bottles; in 410,220 65-count bottles; in 3,451,224 35-count, 65-count and 90-count bottles; in 149,616 35-count and 65-count bottles; in 46,680 50-count bottles; in 129,048 35-count bottles; in 23.856 65-count bottles; and in 11,760 65-count bottles |
|||
|
Introvale tablets (levonorgestrel and ethinyl estradiol), USP 0.15mg/0.03mg, 91/carton, 8,681 cartons |
Sandoz Inc., Broomfield, CO / Laboratorios Leon Farma S.A, Spain for Sandoz Inc., Princeton, NJ |
III |
Contraceptive tablets out of sequence: contraceptive tablets out of sequence: Introvale (levonorgestrel and ethinyl estradiol tablets USP 0.15mg/0.03mg) is volunatrily being recalled due to inactive placebo tablets incorrectly placed in week 9, and active tablets placed in week 13 on the blister card. |
|
Hydrocodone Bitartrate and Acetaminophen Tablets, 7.5 mg/650 mg Tablets, 875 500-count bottles |
Watson Laboratories Inc., Corona, CA |
III |
Defective Container: Defective bottles may not have tamper evident seals properly seated, and therefore it may be difficult to determine if the product had been opened or tampered with. |
|
Acanya (clindamycin phosphate and benzoyl peroxide) Gel 1.2% and 2.5%, 3.5g physician samples, 45,000 tubes |
Valeant Pharmaceuticals, Bridgewater,NJ, Coria Laboratories Division |
III |
Labeling:Incorrect or Missing Lot and/or Exp Date assigned to a lot of physicians samples. |
|
Hydroxychloroquine Sulfate Tablets, USP 200mg, 25,830 100-tablet bottles |
Sandoz Inc., Broomfield, CO |
III |
Failed Tablet/Capsule Specifications: Sandoz is recalling one lot of Hydroxychloroquine Sulfate Tablets, USP, 200mg, due to illegibility of the logo on some tablets. |
|
Paclitaxel Injection, USP 300mg/50mL, 71,129 multiple dose vials |
Fresenius Kabi USA, Lake Zurich, IL / Manufactured in India for APP Pharmaceuticals, Schaumburg, IL |
III |
Labeling: Incorrect or Missing Package insert-missing text on the product insert in the 'Clinical Studies" and "Specific Adverse Events" sections. |
|
Other Product Specifications |
|||
|
Product(s)
|
Recaller / Manufacturer |
Class
|
Reason
|
|
Carboplatin Injection, 600mg/60mL, 23,315 vials, and 450 mg/45mL, 31,326 vials, Cytotoxic Agent, Sterile, Multi-Dose Vials |
Hospira, Inc., Lake Forest, IL / Hospira Inc., Lake Forest, IL , product of Australia |
I |
Crystallization: Product is being recalled due to visible particulates identified during a retain sample inspection. Findings have identified the particles as Carboplatin crystals. |
|
Piperacillin and Tazobactam for Injection, USP 40.5 grams/pharmacy bulk package vial, 37,303 vials |
Apotex Corp., Weston, FL / Hospira Healthcare India Lvt. Ltd., Irungattukottai, India |
I |
Crystallization: Potential to exhibit precipitation/crystallization in IV bag or IV line upon reconstitution. |
|
Piperacillin and Tazobactam for Injection, USP 40.5 grams/bulk package vial, 52,398 vials |
Apotex Corp., Weston, FL / Hospira Healthcare India Lvt. Ltd., Irungattukottai, India |
I |
Crystallization: Potential to exhibit precipitation/crystallization in IV bag or IV line upon reconstitution. |
|
Metronidazole Tablets USP, 500mg packaged in different counts, 25,230 tablets |
Physicians Total Care, Inc. Tulsa, OK / Pliva Krakow Pharma, Krakow, Poland |
II |
Failed Tablet/Capsule Specifications: Some tablets had the potential to not conform to weight specifications. |
|
Abilify (aripiprazole) tablets, 30 mg, 21 blister packs, 100 tablets each |
Bristol Myers Squibb Manufacturing Co., Humacao, PR |
II |
CGMP Deviations: A drum of Abilify 30mg Tablets rejected during the compression stage was not segregated from the other portion of the lot and was inadvertently shipped, packaged and distributed. |
|
Ethambutol tablets USP 400 mg, 18,825 bottles of 90, 100 or 1,000 tablets each |
West-ward Pharmaceutical Corp., Eatontown, NJ |
II |
Discoloration: Ethambutol Tablets USP 400 mg have tablets cores that may be discolored. |
|
Temodar Capsule, 20mg per capsule, 5 capsules per package, 17,169 packages |
Schering-Plough Products, LLC Las Piedras, PR / Schering Corporation, Kenilworth, New Jersey |
II |
Failed Impurities/Degradation Specifications: The recall is being initiated due to an out-of-specification result in the degradation product testing detected during stability monitoring. |
|
Carvedilol Tablets, USP, 12.5 mg 500 count tablets per bottle, 11,508 bottles |
Mylan Pharmaceuticals Inc., Morgantown, WV |
II |
Failed Tablet/Capsule Specifications: Product exceeds specification for tablet weight and tablet thickness |
|
Irinotecan Hydrochloride Injection 40mg/2mL, 23,672 2mL Single Dose Vials |
Fresenius Kabi USA LLC, Schaumburg, IL, mfg for APP Pharmaceuticals LLC, Schaumburg, IL |
II |
Crystallization: Active pharmaceutical ingredient is precipitating in product solution. |
|
Daytrana (methylphenidate) transdermal system patch, delivers 10 mg over 9 hours, 1 patch per pouch, 335,910 patches |
Noven Pharmaceuticals, Inc., Miami, FL |
II |
Defective Delivery System: Out of Specification results for the z-statistic value, which relates to the patients and caregiver ability to remove the release liner from the patch adhesive prior to administration, were obtained. |
|
Meprobamate tablets, USP, 200mg and 400 mg, 100 tablets/bottle, 9,824 bottles |
Watson Laboratories Inc., Corona, CA |
II |
Failed Impurities/Degradation Specifications: Watson Laboratories Inc. is recalling meprobamate because an out of specification result for an impurity -- diphenyl sulfone. |
|
Zarah Drospirenone/Ethinyl Estradiol Tablets, 3.0mg/0.03mg, 28/carton, 136,720 cartons |
Watson Laboratories Inc., Corona, CA |
II |
Failed Tablet/Capsule Specifications: tablet breakage while pushing through the blister pack. |
|
Diclofenac Sodium and Misoprostol Delayed-Release Tablets, 750 mg/0.2 mg, 392,400 tablets in 60-count bottles |
Watson Laboratories. Inc., Davie, FL / Watson Laboratories Inc., Corona, CA |
II |
Failed Tablet/Capsule Specifications: Broken tablets. |
|
Oxcarbazepine Oral Suspension, 300mg/5mL, 10,267 250mL bottles |
Boehringer Ingelheim, Roxane Laboratories Inc., Columbus, OH |
II |
Resuspension Problems: Recalled lot did not meet resuspendability requirements. |
|
Glimepiride Tablets USP, 2mg, 100 tablets per bottle, 10,373 bottles |
Qualitest Pharmaceuticals, Huntsville, AL |
II |
Failed Tablet/Capsule Specifications: One oversized tablet was found in a sealed 100 count bottle of Glimepiride at the retail level. |
|
Ranitidine Syrup (Ranitidine Oral Solution USP) 15 mg/mL, 133,968 bottles |
Actavis South Atlantic LLC, Sunrise, FL / VistaPharm Inc., Birmingham, AL |
II |
Failed Impurity/Degradation Specification; "Related Compound C" |
|
Enablex (darifenacin) Extended Release Tablet, 15mg per tablet, 188,600 seven-tablet physician sample bottles |
Warner Chilcott US LLC., Rockaway, NJ / Novartis Pharma Stein, Stein, Switzerland |
II |
Failed Impurities/Degradation Specifications: unspecified degradation product. |
|
Nimodipine Capsules 30mg, 46,387 cartons of 30- and 100-count blister cards |
Sun Pharmaceutical Industries, Cranbury, NJ |
II |
Crystallization; crystallized nimopdipine. |
|
Ethambutol Hydrochloride Tablets, USP 400mg, 118 60-tablet bottles |
VersaPharm Inc., Marietta, GA / West-ward Pharmaceutical Corp., Eatontown, NJ |
II |
Discoloration: Ethambutol HCI Tablets 400mg is being recalled due to a Out of Specification result for description testing for a surface defect of ink. |
|
Merrem I.V., 1g meropenem equivalent, 81,900 30mL vials |
AstraZeneca Pharmaceuticals, Wilmington, DE / ACS Dobfar SpA Viale Addetta, Tribiano, Milano, Italy |
II |
Presence of Precipitate: potential for incomplete constitution upon addition of diluent. |
|
Pyridostigmine Bromide Tablets, USP, 960 100-tablet bottles |
CorePharma LLC, Middlesex, NJ |
II |
Failed Stability Specifications: Pyridostigmine Bromide tablets, is being recalled due to an out of specification test result during stability testing. |
|
Nystatin Oral Suspension, USP 500,000 units/5mL, 1,707,050 5mL cups |
VistaPharm, Inc., Largo, FL |
II |
Failed Impurities/Degradation Specifications: Test failure of single largest peak at 18 months. |
|
Lisinopril Tablets, USP, 2.5mg, 51,704 100-count and 1,000-count bottles |
Blu Pharmaceuticals, Franklin, KY / West-ward Pharmaceutical Corp., Eatontown, NJ |
II |
Failed Impurities/Degradation Specifications: Out of specification results for individual other unknown related compounds were obtained at the 48 month time-point. |
|
Propranolol Hydrochloride Tablets, USP, 10mg, 9,003 1,000-count bottles |
Teva Pharmaceuticals USA, Sellersville, PA / Teva Czech Industries, sro, Opava komanov, Czech Republic |
II |
Failed Tablet/Capsule Specification: Teva is recalling certain lots of Propanolol HCI Tablets, 10mg due to the potential of some tablets not conforming to weight specification. |
|
Omerprazole Delayed-Release Capsules, USP 20mg, 3,867 1,000-capsule bottles |
Kremers Urban Pharmaceuticals, Princeton, NJ / Kremers Urban Pharmaceuticals, Seymour, IN |
II |
Failed Tablet/Capsules Specifications: some capsules over time do not meet or are suspected to not meet the specification for acid resistance. |
|
Next Choice One Dose Emergency Contraceptive (levonorgestrel tablet), 1.5mg, 1,002,394 tablets, one per blister card |
Watson Laboratories, Inc., Corona, CA |
II |
Failed Tablet/Capsule Specifications: Multiple complaints for push through tablet breakage. |
|
Alprazolam Orally Disintegrating Tablets, USP 2mg, 756 100-count bottles |
Actavis Elizabeth LLC., Elizabeth, NJ |
II |
Failed Tablet/Capsule Specifications; partial tablet erosion resulting in tablet weights below specification in some tablets. |
|
Phenytoin Oral Suspension USP 125mg/5ml 50 cups per case, 27,788 cases |
VistaPharm, Inc., Largo, FL / VistaPharm, Inc., Largo, FL |
II |
Defective Container: A lidding deformity allowed for the product to have out of specification results for assay and viscosity at the 12 month stability timepoint. |
|
Cabergoline Tablets, 0.5mg, 13,232 8-count bottles |
Teva Pharmaceuticals USA, Inc., Sellersville, PA |
III |
Impurities/Degradation Products: This lot of product will not meet the impurity specification over shelf life. |
|
Leukine sargramostim 5x500 mcg/mL vial, 138,505 vials |
Bayer HealthCare Pharmaceuticals, Lynnwood, WA / Bayer HealthCare Pharmaceuticals, LLC Seattle, WA |
III |
Impurities/Degradation Products: A confirmed out of specification result for Leukine sargramostin lot B18827 occurred at the three month time point. |
|
Fougera Ketoconazole Cream 2%, 15g tube, 60,210 units |
Fougera Pharmaceuticals Inc., Melville, NY |
III |
Failed Impurities/Degradation Specifications due to an Out of Specification result for an unknown degradant product. |
|
Major Oral Peroxide (Carbamide Peroxide 10%) 2fl oz, 17,679 bottles |
Axcentria Pharmaceuticals LLC., Telford, PA |
III |
Stability data does not support expiration date: Stability data indicates the Carbamide Peroxide will degrade below acceptable levels within the 24-month expiration date printed on the packaging. |
|
Estee Lauder DayWear Sheer Tint Release, Advanced Multi-Protection Anti-Oxidant Moisturizer Broad Spectrum SPF 15, 241,236 1.7-oz tubes, 544,548 1.5 mL foil packs |
Estee Lauder Inc., New York, NY |
III |
Failed Stability Specifications: The active sunscreen ingredient, avobenzone 3%, may not be stable over the shelf life, the sunscreen effectiveness may be less than labeled. |
|
Perindopril Erbumine Tablets, 8mg, 100 tablets/bottle, 3,553 bottles |
Boehringer Ingelheim Roxane Inc., Columbus, OH / Boehringer Ingelheim, Roxane Laboratories Inc., Columbus, OH |
III |
Impurities/Degradation Products: Out of Specification results found for impurity B, identified as the drug's metabolite. |
|
Femtrace, 5,439 bottles of 0.9mg and 3,155 bottles of 0.45 mg tablets, 100/bottle, 5,439 bottles |
Warner Chilcott Co. LLC, Fajardo, PR / Pharmaceuticals International Inc., Hunt Valley, MD |
III |
Failed Impurity/Degradation Specifications due to moisture ingress in individual bottles |
|
Lac-Hydrin (ammonium lactate) Lotion, 12% in 4,016 400g bottles; and Lac-Hydrin (ammonium lactate) Cream 12%, in 6,535 280g & 385g tubes |
Ranbaxy Inc., Princeton, NJ / Ranbaxy, Jacksonville, FL |
III |
Crystallization: Recall is due to a non-characteristic gritty/sandy texture to the product which is likely due to some crystallization of the product. |
|
Pulmicort Respules 0.25 mg and 0.5 mg per 2 ml Budesonide Inhalation Suspension 30 ampules/carton, 65,179 cartons |
AstraZeneca LP, Westborough, MA |
III |
Does Not Meet Monograph: Budesonide may be slightly above or below the specification range. |
|
Four OTC cough/cold products containing dextromethorphan hydrobromide, guaifenesin and phenylephrine HCl for Walgreens, Rite Aid, Wal-Mart and Select Brands, some 4 oz and some 8 oz, totaling 42,948 bottles |
Aaron Industries Inc., Lynwood, CA |
III |
Presence of Precipitate; white substance confirmed as Guaifenesin, an active ingredient was observed in some bottles. If the product is shaken or warmed the white particles goes into the solution. |
|
Suprax (cefixime) USP, 100mg, 64,368 50mL bottles |
Lupin Pharmaceuticals, Baltimore, MD / Lupin Ltd., Mumbai, India |
III |
Discoloration: Product may not meet specifications for color description once reconstituted. |
|
buPROPion Hydrochloride Extended Release Tablets USP, 100 mg, 100/carton, 389 cartons |
American Health Packaging, Columbus, OH / Actavis South Atlantic LLC |
III |
Failed Stability Specifications; out of specification results at the 9 month stability time point for color, dissolution and related compounds. |
|
Acetaminophen/Dextromethorphan HBr/Phenylephrine HCL 325/10/5 mg, 60,048 blister packs, 20 gel caps in each |
L. Perrigo Co., Allegan, MI |
III |
Failed Stability Specifications: The product had a stability failure at the 12 month time point for the active ingredient Phenylephrine HCL |
|
Ethambutol Hydrochloride Tablets USP 400mg, 4,896 100-count bottles |
Lupin Pharmaceuticals Inc., Baltimore, MD / Lupin Ltd., Mumbai, India |
III |
Failed Impurities/Degradation Specifications: This product is being recalled to an out of specification result for an impurity. |
|
Portia (levonorgestrel and ethinyl estradiol) tablets, USP, 0.15mg 28 tablets per blister card, Jolessa (levonorgestral/ethinyl estradiol) tablets, 0.15mg/0.03mg, 91 tablets per dispenser, 17,661 blister cards and 71,893 dispensers |
Teva Pharmaceuticals USA, Sellersville, PA / Barr Laboratories, Inc., Pomona, NY |
III |
Failed Tablet/Capsule Specifications: This recall is being carried out due to an out of specification result for appearance. |
|
Liothyronine Sodium Tablets, USP, 5mcg 100 count, 21,667 bottles |
L. Perrigo Co., Allegan, MI |
III |
Failed Impurities/Degradation Specifications: Perrigo is conducting a wholesale level recall of two batches of this product due to an out of specification impurity result during three month stability testing. |
|
17,108 blister packs of Seasonale (Levonorgestrel/Ethinyl Estradiol Tablets), 0.15 mg/0.03 mg, 3 extended-cycle tablet dispensers, 91 tablets each |
Teva Pharmaceuticals USA, Sellersville, PA |
III |
Failed Impurity/Degradation Specifications; an impurity identified as N-Butyl-Benzene Sulfonamide (NBBS) detected during impurity testing. |
|
120,049 blister packs of Camrese (Levonorgestrel/Ethinyl Estradiol Tablets), 0.15 mg/0.03 mg and Ethinyl Estradiol 0.01 mg tablets, 2 Extended-Cycle Tablet Dispensers and 1 Extended-Cycle Tablet Dispenser |
Teva Pharmaceuticals USA, Sellersville, PA |
III |
Failed Impurity/Degradation Specifications: an impurity identified as N-Butyl-Benzene Sulfonamide (NBBS) detected during impurity testing. |
|
Six cards of 28 Camila 0.35 mg tablets |
Physicians Total Care Inc., Tulsa, OK / Teva Pharmaceuticals USA, Sellersville, PA |
III |
Failed Impurity/Degradation Specifications: an impurity identified as N-Butyl-Benzene Sulfonamide (NBBS) detected during impurity testing. |
|
6 oral solids recalls *
|
Teva Pharmaceuticals USA, Sellersville, PA / TEVA Pharmaceuticals, Sellersville, PA |
III |
Failed Impurity/Degradation Specification; an impurity identified as N-Butyl-Benzene Sulfonamide was detected during impurity testing. |
|
* Errin (norethindrone tablets, USP) 0.35mg, 6 blister cards, 154,536 cartons; Camila (norethindrone, USP) 0.35 mg Tablets, 6 cards of 28 pills, 59,240 cartons; Lessina (levonorgestrel and ethinyl estradiol tablets) 0.1mg/0.02mg, 28 day regimen, 148,785 packs; Junel Fe (noethindrone acetate and ethinyl estradiol tablets, USP, and ferrous fumarate tablets) 1.5/30, 28 day regimen, 47,200 packs; Jolessa (levonorgestrel/ethinyl estradiol tablets, USP) 0.15mg/0.03mg, 91 day regimen 54,412 packs; and Balziva (norethindrone and ethinyl estradiol tablets) 0.15mg/0.03mg, 28 day regimen, 36,708 packs |
|||
|
Rite Aid brand Maximum Strength Tussin Cough & Cold Mucus Relief CF, (dextromethorphan HBr 10 mg, guaifenesin 200 mg and phenylephrine HCl 5 mg), 27,144 4 oz. bottles |
Aaron Industries Inc., Lynwood, CA |
III |
Presence of Precipitate; white substance confirmed as Guaifenesin, an active ingredient was observed in some bottles. If the product is shaken or warmed the white particles goes into the solution. |
|
Glenmark Gabapentin Tablets, 600 mg and 800 mg, 52,416 500-count bottles; Glenmark Pravastatin Sodium Tablets, 40 mg, 246,528 90-count bottles; and Glenmark Topiramate Tablets, 200 mg, 9,624 60-count bottles. |
Glenmark Generics Inc. Mahwah, NJ / Glenmark Generics Ltd., Ltd. Colvale-Bardez, India |
III |
Chemical Contamination: Gabapentin Sodium tablets is recalled due to complaints related to an off-odor described as moldy, musty or fishy in nature. |
|
Amoxicillin for Oral Suspension, USP 400mg/5mL , 758,554 bottles |
Teva Pharmaceuticals USA, Sellersville, PA |
III |
Discoloration: This recall is being carried out due to a yellow to brown discolored Amoxicillin powder on the inner foil seal of the bottles. |
|
Nicardipine Hydrochloride Injection 25mg/10mL , 33,840 10-mL single-use vials |
West-ward Pharmaceutical Corp., Eatontown, NJ / Exela Pharma Sciences, LLC, Lenoir, N.C. |
III |
Failed Impurity/Degradation Specifications; out of specification value for impurity Nitrophenylpuridine Derivative. |
|
Folic Acid Injection, USP 5mg/mL, 80,480 10-mL multi-dose vials |
Fresenius Kabi USA, Lake Zurich, IL / APP Pharmaceuticals Schaumburg,IL |
III |
Failed Impurities/Degradation Specification |
|
Dr. Sheffield's Triple Antibiotic Ointment Bacitracin 400 units, Neomycin Sulfate 3.5mg, Polymyxin-B Sulfate, 105,744 tubes |
Faria Ltd., dba Sheffield Pharmaceuticals, New London, CT |
III |
Failed Stability Specifications: Unable to meet shelf life expiry. |
|
Fentanyl Transdermal System, 50mcg/h, 98,952 boxes |
Actavis, Salt Lake City, UT / Watson Laboratories Inc., Corona, CA |
III |
Failed Impurities/Degradation Specifications. |
|
Medroxyprogesterone acetate, micronized API in 6.0 kg drums, 194.62 kg |
Bayer HealthCare Pharmaceuticals, Wayne, NJ / Bayer Schering Pharma AG, Berlin, Germany |
III |
Failed Stability Specifications: Out of specification results for particle size were obtained at the 60 month test point. |
|
Amoxicillin for Oral Suspension USP 200mg/5mL, 109,080 75mL and 100mL bottles |
Teva Pharmaceuticals USA, Sellersville, PA / Teva Canada Ltd., Toronto, Ontario, Canada |
III |
Discoloration: This recall is being carried out due to an orange to brown discolored Amoxicillin powder on the inner foil seal of the bottles. This is an expansion of RES 65050. |
|
Tagitol V Barium Sulfate Suspension (40% w/v, 30% w/w), 3,096 20mL bottles |
Bracco Diagnostics Inc., Monroe Township, NJ / E-Z-EM Canada Inc. unit of E-Z-EM, Inc., Lake Success, NY |
III |
Failed Stability Specifications: Tagitol V Barium Sulfate Lot sampled at 10 months exhibited results above the upper specification for viscosity. |
|
Loratadine, USP 10mg Antihistamine packaged in 11,846,608 blister packs and sold under seven brands |
Novartis Consumer Health., Lincoln, NE |
III |
Failed Tablet/Capsule Specifications: The products are being recalled due to chipped and broken tablets. |
|
Bumetanide Tablets USP 0.5mg, 11,496 100-count bottles and 62 500-count bottles |
Sandoz Inc., Wilson, NC |
III |
Failed Moisture Limit; Out of Specification (OOS) results were obtained for moisture content during stability testing at the12 month time point, controlled room temperature conditions. |
|
Folic Acid Injection, USP 5mg/ml, 215,340 10-mL multi-dose vials |
Fresenius Kabi USA, Lake Zurich, IL / APP Pharmaceuticals Schaumburg,IL |
III |
Failed Impurities/Degradation specifications: out-of-specification results at the 14 & 15 month time point. |
|
Potency / Content Uniformity |
|||
|
Product(s)
|
Recaller / Manufacturer |
Class
|
Reason
|
|
Hydrocodone bitartrate and Acetaminophen tablets 10mg/500mg, packaged in 30,60,90,100,120,150,180,500,1000 count bottles, 897,379 bottles |
Vintage Pharmaceuticals LLC DBA Qualitest Pharmaceuticals, Huntsville, AL |
I |
Superpotent (Multiple Ingredient) Drug: Complaint received of oversized tablets. |
|
Hydrocodone bitartrate and Acetaminophen tablets 10mg/500mg 100 tablets per carton, 3,407 cartons |
Mylan Institutional Inc. (dba UDL Laboratories), Rockford, IL / Qualitest Pharmaceuticals, Huntsville, AL |
I |
Superpotent (Multiple Ingredient) Drug: Oversized tablets resulting in superpotent assays of both the hydrocodone and acetaminophen components. |
|
Mylan, Tacrolimus Capsules 0.5 mg, 15,181 100-count bottles |
Mylan Pharmaceuticals Inc., Morgantown, WV / Mylan LLC, Caguas, Puerto Rico |
II |
Failed USP Content Uniformity Requirements: OOS result reported on retained samples. |
|
Thyro-Tab 0.050 mg., packaged in 150,000-tablet bulk drums for repackaging, 3,789,426 tablets |
Lloyd Inc. of Iowa, Shenandoah, Iowa |
II |
Subpotent; 9-month stability interval |
|
Levothroid (levothyroxine sodium tablets, USP), 50 mcg, 37,441 100-count bottles |
Forest Pharmaceuticals, Inc., Subsidiary of Forest Laboratories, Inc., St. Louis, MO / Lloyd Inc. of Iowa, Shenandoah, Iowa |
II |
Subpotent; 9-month stability interval |
|
Thyro-Tab 0.075 mg., packaged in 150,000-tablet bulk drums for repackaging, 1,924,133 tablets |
Lloyd Inc. of Iowa, Shenandoah, Iowa |
II |
Subpotent; 9-month stability interval |
|
Levothroid (levothyroxine sodium tablets, USP), 75 mcg, 16,548 100-count bottles |
Forest Pharmaceuticals, Inc., Subsidiary of Forest Laboratories, Inc., St. Louis, MO / Lloyd Inc. of Iowa, Shenandoah, Iowa |
II |
Subpotent; 9-month stability interval |
|
Levothyroxine Sodium Tablets, USP, 175 mcg, 7,432 90-count bottles |
Alara Pharmaceutical Corp, San Juan, PR / Patheon Puerto Rico Inc. |
II |
Subpotent; 15 month stability |
|
Exparel (bupivacaine liposome injectable suspension) 1.3%, 266 mg/20mL single use vials, investigational, 324 vials |
Pacira Pharmaceuticals, Inc., San Diego, CA / Paelra Pharmaceuticals Inc., San Diego, CA |
II |
Subpotent; bupivacaine |
|
Clonazepam Orally Disintergrating Tablets, USP, 0.5mg, 60/box, three boxes |
Teva Pharmaceuticals USA, Inc., Sellersville, PA |
II |
Failed Content Uniformity Specifications: Recall is being carried out due to an out-of-specification result for content uniformity. |
|
Rifadin (rifampin) capsules, USP, 150mg, 998 100-count bottles |
Sanofi-Synthelabo, Laval, Quebec, Canada |
II |
Subpotent Drug: During review of retain samples, the manufacturer observed low fill in some capsules, which was related to an issue detected with the encapsulating equipment. |
|
Rifadin, Rifampin capsules, 150mg, 2,462 30-count bottles |
Sanofi-Aventis US, Bridgewater, NJ |
II |
Subpotent drug: low fill volume in some of the capsules. |
|
Ethambutol Hydrochloride Tablets, USP 100mg, 100 count tablets, 3,061 bottles |
West-ward Pharmaceutical Corp., Eatontown, NJ / West-ward Pharmaceutical Corp., Eatontown, NJ, for VersaPharm Inc., Marietta, GA |
II |
Subpotent Drug: Out of Specification results for assay at the stability time-point of 24 months. |
|
Cetacaine Liquid Topical Anesthetic, 18,941 14g and 30g bottles |
Cetylite Industries Inc., Pennsauken, NJ |
II |
Subpotent Drug: Cetacine Topical Anesthetic Liquid is being recalled because one of the three active ingredients, tetracaine, is below the specification limit at the 21 month stability test point. |
|
11 oral solids recalls *
|
King Legacy, Bristol, TN, a wholly owned subsidiary of Pfizer / King Pharmaceuticals, Inc., Bristol, TN |
II |
Subpotent Drug: The products were below specification for potency at the expiry stability point. |
|
* 51,591 100-count and 1,000-count bottles of Levoxyl (levothyroxine sodium) tablets, USP, 25 mcg; 195,699 100-count and 1,000-count bottles of Levoxyl (levothyroxine sodium) tablets, USP, 50 mcg; 194,142 100-count and 1,000-count bottles of Levoxyl (levothyroxine sodium) tablets, USP, 75 mcg; 112,914 100-count and 1,000-count bottles of Levoxyl (levothyroxine sodium) tablets, USP, 88 mcg; 195,201 100-count and 1,000-count bottles of Levoxyl (levothyroxine sodium) tablets, USP, 100;107,236 100-count and 1,000-count bottles of Levoxyl (levothyroxine sodium) tablets, USP, 112 mcg;103,796 100-count and 1,000-count bottles of Levoxyl (levothyroxine sodium) tablets, USP, 125 mcg;77,272 100-count and 1,000-count bottles of Levoxyl (levothyroxine sodium) tablets, USP, 137 mcg; 56,405 100-count and 1,000-count bottles of Levoxyl (levothyroxine sodium) tablets, USP, 150 mcg; 51,438 100-count and 1,000-count bottles of Levoxyl (levothyroxine sodium) tablets, USP, 175 mcg; and 33,227 100-count and 1,000-count bottles of Levoxyl (levothyroxine sodium) tablets, USP, 200 mcg. |
|||
|
Actiq (oral transmucosal fentanyl citrate) 200 mcg, lozenge on a stick in single blister packs and 30-count cartons -- 316 cartons in all |
Cephalon, Inc., Salt Lake City, UT / Teva Pharmaceuticals USA, Sellersville, PA |
II |
Subpotent Drug: Product is being recalled due to an out of specification dissolution result during stability testing. |
|
Oxytocin Injection, USP (synthetic), 10 USP units/mL, 221,600 1-mL vials |
Fresenius Kabi USA, Lake Zurich, IL / APP Pharmaceuticals Schaumburg,IL |
II |
Subpotent Drug; 15-month stability test station. |
|
Valacyclovir HCI Tablets, 500mg, 100 tablets per carton, 1,536 cartons |
American Health Packaging, Columbus, OH |
II |
Subpotent |
|
Lidocaine Hydrochloride 2% and Epinephrine 1:100,000 Injection for dental block and infiltration, 12,497 boxes of 50 cartridges each |
Novocol Pharmaceutical of Canada, Cambridge, Canada / Novocol Pharmaceutical of Canada, Cambridge, Ontario, Canada |
II |
Subpotent Drug; Two lots of Lidocaine 2% with Epinephrine 1:100,000 Injectables, distributed under the names: Octocaine 100, and 2% Xylocaine Dental, may be subpotent for the epinephrine component. |
|
Irinotecan Hydrochloride Injection, 40mg/2mL, 9,020 2mL single-dose vials; Irinotecan Hydrochloride Injection, 100mg/5mL, 9,813 5mL single-dose vials |
West-ward Pharmaceutical Corp., Eatontown, NJ / Thymoorgan Pharmazie GmbH, Vienenburg, Germany |
II |
Superpotent Drug: a recent review of the USP revealed that an incorrect calculation was used to determine the amount of irinotecan to use in formulation which will result in an assay higher than the labeled claim. |
|
Ketoconazole Shampoo, 2%, 391,055 bottles |
Sandoz Inc., Princeton, NJ / Tolmar Inc., Fort Collins, CO |
II |
Subpotent |
|
Derma-Smoothe/FS (fluocinolone acetonide), 0.01% Topical Oil, two lots, 8,736 bottles and 8,592 4fl oz bottles |
Hill Dermaceuticals, Inc., Sanford, FL |
III |
Subpotent; 12 month stability timepoint |
|
Glycopyrrolate Tablets, USP, 1mg 100 tablets per bottle, 1,185 bottles |
West-Ward Pharmaceutical Corp., Eatontown, NJ |
III |
Subpotent Drug: Test results for assay were confirmed Out Of Specification (OOS) at 92.8% (specification of 97.0 - 103.0%). |
|
Ventavis (iloprost) Inhalation Solution, 10mcg/1mL, Sterile, 30 single use ampules/carton, three cartons |
Actelion Pharmaceuticals U.S, Inc. South San Francisco, CA |
III |
Subpotent Drug: OOS assay result at the 12 month stability time point. |
|
Sodium Sulfacetamide 10% and Sulfur 5% Lotion , 8,437 bottles |
Austin Pharmaceuticals LLC., Wilmington, DE / Mission Pharmacal Co., Boerne, TX |
III |
Superpotent: Drug product active ingredients were formulated incorrectly (too high) with respect to the label strength. |
|
Acetic Acid Otic Solution, USP, 15mL/bottle, 11,472 bottles |
Hi-Tech Pharmacal Co., Inc, Amityville, NY |
III |
Subpotent Drug: The product/lot is out-of-specification for the assay of acetic acid at the 18 month test station. |
|
Cherry Cepacol Sore Throat Lozenges, Benzocaine 15 mg Menthol 3.6mg, 16- and 576-count, 35,269 cases |
Reckitt Benckisser, Parsippany, NJ |
III |
Subpotent Drug: Product did not conform to the 18-month stability test specification for active Free Benzocaine. |
|
Oxcarbazepine Tablets, 150mg, in 8,550 100-count cartons and bottles |
Boehringer Ingelheim, Roxane Laboratories Inc., Columbus, OH |
III |
Subpotent Drug: The firm discovered out of specification results for assay and the extended investigation revealed the potential for lower weight tablets. |
|
Bucalsep Oral Solution (Benzocaine, 600mg, Cetylpyridinum Chloride 30mg, Menthol 45 mg Zinc Chloride 30mg) 1Fl Oz., 10,440 bottles |
Hi-Tech Pharmacal Co., Inc, Amityville, NY / Manufactured for Gil Pharmaceutical Corp., Ponce, Puerto Rico |
III |
Subpotent; Cetylpyridinum Chloride |
|
HydrOXYzine Hydrochloride Oral Solution, USP, 10mg/5mL , 15,240 bottles |
Hi-Tech Pharmacal Co. Inc., Amityville, NY |
III |
Subpotent; 24 month stability test station. |
|
Derma-Smoothe/FS (fluocinolone acetonide), topical scalp oil 0.01%, 4 fl oz, 8,808 bottles |
Hill Dermaceuticals, Inc., Sanford, FL |
III |
Subpotent Drug: the active ingredient, fluocinolone acetonide, was found to be subpotent during the firm's routine testing. |
|
LA MER the spf 18 fluid tint, broad spectrum spf 18, 1.7fl oz, 49,845 tubes each of light, medium and dark shades |
Max Huber Research Labs, New York, NY / Made in Belgium |
III |
Subpotent Drug; Avobenzone 3%, one of the active sunscreen ingredients may be subpotent and sunscreen effectiveness may less than labeled. |
|
Duet DHA Balanced, Duet DHA 400, Duet DHA 400 ec, Duet DHA 430, Duet DHA 430 ec, NataChew and Nata Komplete, comprising various combinations of multivitamin tablets and Omega-3 softgel capsules in 64,494, 4,450, 2,240, 5,895, 2,985 and 1,492 bottles and 5,941 physician samples |
Stayma Consulting Service, Suwanee, GA, for Eckson Labs and W.H. Nutritionals, both of Wilmington, DE |
III |
Subpotent; Beta carotene (Vitamin A). |
|
Oxycodone and Acetaminophen Capsules, USP 5mg/500mg1,260 100-count and 500-count bottles |
Watson Laboratories Inc., Corona, CA |
III |
Subpotent drug: Two lots of Oxycodone and Acetaminophen Capsules, USP 5/500mg are being recalled due to low out of specification (OOS) assay result obtained at expiry (24 months) for the oxycodone portion of the product. |
|
Giltuss Antitussive Expectorant and Nasal Decongestant and Exactuss Antitussive Expectorant and Nasal Decongestant, 50,848 1oz, 8oz and 16oz bottles |
Gil Pharmaceutical Corp., Puerto Rico / Hi-Tech Pharmacal Co., Amityville, NY |
III |
Subpotent; phenylephrine HCI |
|
Derma-Smoothe/FS (fluocinolone acetonide) 0.01% Topical Oil, two groups of lots |
Hill Dermaceuticals, Inc., Sanford, FL |
III |
incorrect/ Undeclared Excipients: NyQuil Liquid Original bottles were inadvertently overwrapped with NyQuil Liquid Cherry information as a result the outer wrap does not correctly identify color additives, particularly FD&C Yellow No. 6 and FD&C Yellow 10 |
|
Ciclopirox Shampoo 1%, 850 120mL bottles |
Taro Pharmaceuticals, Hawthorne, NY / Taro Pharmaceuticals Inc., Brampton, Ontario, Canada |
III |
Subpotent drug |
|
Compliance with NDA / Monograph Requirements |
|||
|
Product(s)
|
Recaller / Manufacturer |
Class
|
Reason
|
|
30,000 Actra-SX 500 Capsules, Maximum Strength, Energizer |
Body Basics, Canoga Park, CA |
I |
Marketed without an Approved NDA/ANDA; product contains sildenafil, an active ingredient in a FDA approved product for the treatment of erectile dysfunction. |
|
Blue Male Enhancement Pill, 50,000 capsules |
The Menz Club, Ridgeland, MS |
I |
Marketed without an Approved NDA/ANDA; product found to contain sulfoaildenafil, an analogue of sildenafil, the active ingredient in a FDA approved product used for erectile dysfunction, making it an unapproved new drug. |
|
Undeclared Tadalafil in 8,000 blister packs of Firminite 2, 4 and 10 count capsules, Libidron 2, 4 and 10 count capsules, and 1,000 Extra Strength Instant Hot Rod 4 and 10 count capsules. blister packs. |
InSo-Independent Contractor for West Coast Nutritionals, Allen, TX |
I |
Marketed Without an Approved NDA/ANDA: These products found to contain undeclared tadalafil. Tadalafil is an FDA-approved drug for the treatment of male Erectile Dysfunction, making these products unapproved new drugs. |
|
Mojo Nights and Mojo Nights for Her, 1,000 one-capsule blister packs |
Evol Nutrition, Kennesaw, GA |
I |
Marketed Without an Approved NDA/ANDA: The products were found to contain FDA approved ingredients and analogues of FDA approved ingredients used to treat male erectile dysfunction, making them unapproved new drugs. |
|
Slim Xtreme Herbal Slimming Capsule , 40,400 30-count bottles |
Globe All Wellness, LLC, Hollywood, FL |
I |
Marketed Without an Approved NDA/ANDA: Product tested positive for Sibutramine, an appetite suppressant that was withdrawn from the U.S. market in October 2010 for safety reasons, making this product an unapproved new drug. |
|
Classic Zi Xiu Tang, Bee Pollen Capsules, 60/bottle, 6,048 bottles; and Ultimate Formula capsules, 48/bottle, 16,126 bottles |
Zi Xiu Tang Success, LLC, Kutztown, PA |
I |
Marketed Without an Approved NDA/ANDA: Products were found to contain sibutramine, a previously approved FDA drug removed from the U.S. marketplace for safety reasons, making these products unapproved drugs. |
|
WOW, Health Enterprises, Dietary Supplement (methocarbamol, dexamethasone, diclofenac), 30 caplets/bottle, 17,555 bottles |
Brower Enterprises Inc., Canton, SD / Riger Natural, S.A. Mexico |
I |
Marketed without an Approved NDA/ANDA: Brower Enterprises Inc., is recalling its WOW Health Enterprises Dietary Supplement, because it contains undeclared drug ingredients making it an unapproved drug. FDA sample analysis has found the product to contain methocarbamol, dexamethasone, and diclofenac. |
|
610,000 libigrow capsules in 1,5 and 10 count blister packs; 686,000 libigrow XXXTREME Capsules in 1,5, and 10 count blister packs; 213,000 capsules of BLUE Diamond Pill, supplied in 1, 5 and 10 count blister packs; 1,000 BLUE Diamond Platinum Capsules in 1,5 and 10 count blister packs; 219,000 Mojo nights Capsules in 1 and 5 count blister packs; 70,000 Mojo nights SUPREME Capsules; and 96,000 CASANOVA Capsules. |
Performance Plus Marketing, Inc, Commerce, CA |
I |
Marketed Without an Approved NDA/ANDA: product may contain undeclared slidenafil, tadalafil and analogues of these FDA approved active ingredients, making them unapproved drugs. |
|
Slimdia Revolution capsules, 30-count capsules, unknown quantity |
P&J Trading Co, Fullerton, CA / Yerba Naturals, Buena Park, CA |
I |
Marketed Without an Approved NDA/ANDA: All lots of the dietary supplement Slimdia Revolution are being recalled because they contain sibutramine, a previously approved FDA drug removed from the U.S. marketplace for safety reasons, making it an unapproved new drug. |
|
Super Power capsules, Proprietary Blend 570 mg (with undeclared sildenafil), packaged in 40,480 2-capsules blister packs |
D& S Herbals, LLC, Woodbridge, NJ |
I |
Marketed Without an Approved NDA/ANDA: This dietary supplement has been found to contain sildenafil, an FDA approved drug for the treatment of male erectile dysfunction making this an unapproved new drug. |
|
Reumofan Plus, 30-count tablets per bottle, 586 bottles |
Reumofan Plus USA., Springfield, PA / Riger Natural, S.A. Mexico |
I |
Marketed without an approved NDA/ANDA: Product may contain undeclared active pharmaceutical ingredients Diclofenac Sodium, Dexamethasone, and Methocarbamol. |
|
Maxiloss Weight Advanced, 225 mg proprietary blend of herbs (sibutramine), 600 boxes |
Olaax International, Bartow, FL |
I |
Marketed Without an approved NDA/ANDA: product contains sibutramine, a previously approved FDA drug removed from the U.S. marketplace for safety reasons, making it an unapproved new drug. |
|
Night Bullet Capsules, supplied in 1 count packets, 429,619 capsules |
Green Planet Inc., Riverside, CA |
I |
Marketed Without an Approved NDA/ANDA; product contains analogues of slidenafil and tadalafil which are active pharmaceutical ingredients in FDA-approved drugs used to treat erectile dysfuncation making this product an unapproved new drug. |
|
Rock-it Man Capsules, male sexual stimulant packaged in 11,424 blister cards, each with one or two capsules |
Consumer Concepts, Inc., Roscoe, IL |
I |
Marketed Without an Approved NDA/ANDA: FDA analysis found the product to contain hydroxythiohomosildenafil, an analogue of slidenafil which is an ingredient in an FDA-approved drug for the treatment of male erectile dysfunction, making this an unapproved new drug. |
|
Affirm XL Nutritional Supplement, 400 tablets in 1 and 10 count blister packs and single-pill packs |
Affirm XL, Inc., Ladera Ranch, CA / Dongseo Biopharm Corp., Yongin City, Kyonggi do, South Korea |
I |
Marketed Without an Approved NDA/ANDA: FDA analysis found the product to contain sulfoaidenafil, an analogue of sildenafil which is an ingredient in an FDA-approved drug for the treatment of male erecticle dysfunction, making this an unapproved new drug. |
|
673,851 SexVoltz All Natural Herbal Supplement capsules and 145,632 Velextra All Natural Herbal Supplement capsules packaged in one- and two-capsule blister packs and four-, 10- and 12-capsule bottles |
BeaMonstar Products, Queen Creek, AZ |
I |
Marketed Without an Approved NDA/ANDA; products have been found to contain tadalafil, the active ingredient in an FDA approved product used to treat erectile dysfunction, making this product an unapproved drug. |
|
Lightning ROD capsules, 500mg and 550mg/capsule, 3,720 capsules |
Chang Kwung Products, Woodland Hills, CA |
I |
Marketed Without An Approved NDA/ANDA: Lightning Rod capsules are being recalled because FDA analysis found it to contain an undeclared analogue of sildenafil. Sildenafil is the active ingredient in an FDA-approved product indicated for the treatment of m |
|
Bethel 30 Capsules, packaged in 18,678 30-count bottles |
Bethel Nutritional Consulting, Inc., New York, NY |
I |
Marketed Without an Approved NDA/ANDA: FDA analysis found the product to contain hydroxythiohomosildenafil, an analogue of slidenafil which is an ingredient in an FDA-approved drug for the treatment of male erectile dysfunction, making this an unapproved |
|
Volcano Male Enhancement Liquid, 70 2FL oz bottles |
Myson Corporation Inc., Long Beach, CA |
I |
Marketed Without an Approved NDA/ANDA: All lots of the dietary supplement Slimdia Revolution are being recalled because they contain sibutramine, a previously approved FDA drug removed from the U.S. marketplace for safety reasons, making it an unapproved |
|
Volcano Male Enhancement Capsules 1500mg, 1 count capsules |
Myson Corporation Inc., Long Beach, CA |
I |
Marketed Without an Approved NDA/ANDA: All lots of the dietary supplement Slimdia Revolution are being recalled because they contain sibutramine, a previously approved FDA drug removed from the U.S. marketplace for safety reasons, making it an unapproved |
|
B-50 Capsules, an unknown quantity of 100-count bottles; and Multi-Mineral capsules, an unknown quantity of 200-count bottles |
Healthy Life Chemistry Inc., Deer Park, NY |
I |
Marketed Without An Approved NDA/ANDA: FDA analysis of this product found it to contain undeclared steroids and steroid-like substances making this an unapproved new drug. |
|
Quick Thin capsules, 9,121 30-count bottles |
Bethel Nutritional Consulting, Inc., New York, NY |
I |
Marketed Without An Approved NDA/ANDA: FDA analysis detected the presence of sibutramine, N-Desmethylsibutramine and phenolphthalein. Sibutramine and phenolphthalein were previously available drug products but were removed from the U.S. market making these products unapproved new drugs. |
|
Bethel Advance capsules, 13,266 bottles of 30 capsules each |
Bethel Nutritional Consulting, Inc., New York, NY |
I |
Marketed Without An Approved NDA/ANDA: FDA analyses detected the presence of phenolphthalein, N-di-Desmethylsibutramine, and trace amounts of sibutramine and N-Desmethylsibutramine. Sibutramine and phenolphthalein were previously available drug products but were removed from the U.S. market making these products unapproved new drugs. |
|
J.R. Jack Rabbit All Natural Herbal Supplement, 1,000 tablets |
Jack Rabbit, Inc., Melbourne, FL |
I |
Marketed Without an Approved NDA/ANDA: Laboratory analysis conducted by the FDA has determined that J.R. Jack Rabbit Male Enhancement product was found to contain two undeclared active pharmaceutical ingredients: slidenafil and tadalafil. |
|
Evil Root 1200mg, supplied in 6 capsules |
Fabscout Entertainment Inc., Ft Lauderdale, FL / Tibet Health Medicine Co., Lhasa, Tibet, China |
I |
Marketed Without an Approved NDA/ANDA: product found to contain undeclared slidenafil . |
|
72HP Maximum Potency Male Sexual Enhancement, 578 blister packs of one capsule each |
Fabscout Entertainment Inc., Ft Lauderdale, FL |
I |
Marketed Without an Approved NDA/ANDA: product found to contain undeclared slidenafil . |
|
PRO Power Max Natural Energy Enhancer, 18 blister packs of one capsule each |
Fabscout Entertainment Inc., Ft Lauderdale, FL |
I |
Marketed Without an Approved NDA/ANDA: product found to contain undeclared slidenafil and tadalafil. |
|
Piracetam Cognition Enhancing Agent Dietary Supplement 800mg, Piracetam Powder Cognition Enhancing Agent Dietary Supplement CTD 500 grams, Aniracetam Fast Acting Nootropic Dietary Supplement 750mg capsules, 4,401 bottles, each containing 60 or 240 capsules, or 500 grams of powder |
AB Nutrition, Inc., Spring, TX |
II |
Marketed Without an Approved NDA/ANDA: The product is an unapproved new drug. |
|
7 oral solids recalls *
|
Palmer Natural Products, Star, ID |
II |
Marketed Without an Approved NDA/ANDA: The products are unapproved drugs. |
|
* 14,076 bottles of seven products: Aniracetam & Choline capsules, 430mg Aniracetam 88mg Choline 30, 60,90, and 180 count bottle; Nootropa Cognitive Enhancement Supplement, 400 mg Aniracetam Capsules 60 count bottle; Aniracetam and Choline, Aniracetam 460mg, Choline 76mg, 180 capsules; Piracetam & Choline, 390mg Piracetam 104mg Choline Capsules 30 count bottles, 100 count bottle; Brain Defogger, Piracetam 390mg Choline 104mg capsules, 100 count bottle; Piracetam 390mg Choline 104mg capsules, 250 count bottle; and Oxiracetam & Choline, Oxiracetam 460mg Choline 110mg capsules 30, 60 and 90 count bottles |
|||
|
INK-EEZE Tattoo Numbing Spray, 2 oz., Active Ingredients: Lidocaine HCI 5% Topical Anesthetic, 683 bottles; INK-EEZE Tattoo Black Label Numbing Spray, 8 mL and 59 mL, 2,849 bottles |
Indelicare LLC., Newbury Park, CA |
II |
Marketed without an approved NDA/ANDA: INK-EEZE Tattoo Numbing Spray contains 5% Lidocaine and is being marketed without an approved INDA/ANDA. Lidocaine 5% is an ingredient in many FDA approved products, making Ink-Eeze an unapproved new drug. |
|
Aldactone (spironolactone) tablets, USP 100mg, 31 100-count bottles |
Pfizer Pharmaceutical Group, New York, NY |
II |
Marketed Without an Approved NDA/ANDA: This recall is being initiated because of changes to the dissolution profile in distributed lots resulting from a manufacturing site change. There is currently no approved application supporting the alternate manufacturing site. |
|
Spironolactone tablets, USP 25mg, 2,796 500-count bottles; USP 50mg, 4,848 100-count bottles |
Greenstone LLC., Peapack, NJ |
II |
Marketed Without an Approved NDA/ANDA: This recall is being initiated because of changes to the dissolution profile in distributed lots resulting from a manufacturing site change. There is currently no approved application supporting the alternate manufac |
|
Dissolution |
|||
|
Product(s)
|
Recaller / Manufacturer |
Class
|
Reason
|
|
Quetiapine Fumarate Tablets 25mg, 19,060 100-tablet blister cards |
American Health Packaging, Columbus, OH |
II |
Failed Dissolution Test Requirements: During analysis of long term stability studies at 3 months time point, an OOS was reported for Quetiapine Fumarate Tablets, 25mg. |
|
Propranolol HCl Extended-release capsules, USP, 80 mg, 100/bottle, 60 bottles |
Upsher-Smith Laboratories, Inc., Minneapolis, MN |
II |
Failed Dissolution Test Requirements. |
|
Dextroamphetamine Sulfate Extended Release Capsules, 5mg, 90 capsules per bottle, three lots involving 1,176, 12,948 and 10,968 bottles |
Amedra Pharmaceuticals LLC, Horsham, PA / Catalent Pharma Solutions for CorePharma, Middlesex, NJ |
II |
Failed Dissolution Specifications: The affected lots may not meet the specifications for dissolution over the product shelf life. |
|
Isoniazid Tablets 300 mg USP, 30 and 100 tablets per bottle, 77,531 bottles |
VersaPharm Inc., Marietta, GA / West-ward Pharmaceutical Corp., Eatontown, NJ |
II |
Failed Dissolution Specifications; 36 month stability timepoint. |
|
Taztia XT (diltiazem HCl) USP, Once-A-Day Dosage 360mg, 90 capsules/bottle, 3,040 bottles |
Watson Pharmaceuticals, Parsippany, NJ / Watson Laboratories, Inc., Corona, CA |
II |
Failed Dissolution Specification: Out of a specification result occurred during the 3-month stability testing. Dissolution result at the 4 hour time point was 41% (specification: 20-40%). |
|
Methylphenidate Hydrochloride Extended-Release Capsules, 100-count bottles totaling 16,293 for 20mg; 13,478 for 30 mg and 7,579 for 40 mg |
Teva Pharmaceuticals USA, Sellersville, PA / Teva Pharmaceuticals USA |
II |
Failed Dissolution Specifications: Product is being recalled due to out of specification dissolution results obtained during routine stability testing. |
|
Extended Phenytoin Sodium Capsules, USP 100mg, 895 1,000-count bottles |
Amneal Pharmaceuticals of NY LLC, Hauppauge, NY |
II |
Failed dissolution specifications; 18 month CRT. |
|
buPROPion Hydrochloride Extended-Release Tablets 300mg, 14,856 30-tablet bottles |
Actavis Pharmaceuticals, Morristown, NJ |
III |
Failed Dissolution Specifications: 8 hours for the 18-month stability testing point. |
|
Dextroamphetamine Saccharate, Amphetamine Aspartate Monohydrate, Dextroamphetamine Sulfate, Amphetamine Sulfate Extended-Release Capsules, 15 mg, Class II, 100/bottle, 9,264 bottles |
Actavis Elizabeth LLC, Elizabeth, NJ |
III |
Failed Dissolution Specification: The product lot exceeded specifications |
|
buPROPion Hydrochloride Extended-Release Tablets XL 300mg 30 count tablets, 555,672 bottles |
Actavis South Atlantic LLC Sunrise, FL |
III |
Failed USP Dissolution Test Requirements: Out-of-specification dissolution results at the 8 hour stability testing point. |
|
Budesonide Capsules, 3 mg, Enteric Coated, 1,621 boxes of 30-capsule blister packs |
Mylan Institutional Inc., DBA UDL Laboratories, Rockford, IL / Mylan Pharmaceuticals Inc., Morgantown, WV |
III |
Failed Dissolution Specifications: Routine stability testing at the 12-month interval yielded an out-of-specification (OOS) result for dissolution testing. |
|
Norpace CR (disopyramide phosphate) extended-release capsules USP, 150 mg, 5,410 100-count and 500-count bottles |
Pfizer Inc., Peapack, NJ |
III |
Failed Dissolution Specification. |
|
Prempro (conjugated estrogen/medroxyprogesterone) tablets 0.625mg/2.5mg and 5.0 mg, 432,657 28-tablet blister cards; and 135,520 blister cards of Prempro tablets 0.625mg/5.0mg |
Pfizer Inc., New York, NY / Wyeth Pharmaceuticals Inc., Philadelphia, PA |
III |
Failed Dissolution Specifications: Pfizer Inc is recalling PREMPRO (conjugated estrogens / medroxyprogersterone acetate tablets) because certain lots for this product may not meet the dissolution specification for conjugated estrogens. |
|
135, 520 Prempro tablets 0.625mg/5.0mg, 1 blister card |
Pfizer Inc., New York, NY / Wyeth Pharmaceuticals Inc., Philadelphia, PA |
III |
Failed Dissolution Specifications: Pfizer Inc is recalling PREMPRO (conjugated estrogens/medroxyprogersterone acetate tablets) because certain lots for this product may not meet the dissolution specification for conjugated estrogens. |
|
BuPROPion Hydrochloride Extended-Release Tablets 300mg, 30 per bottle, 15,132 bottles |
Actavis Inc., Parsippany, NJ |
III |
Failed Dissolution Specifications: Dissolution test results at 8 hour time-point were above approved specification limits. |
|
buPROPion Hydrochloride Extended Release Tablets, 300mg, 428,268 30-count bottles |
Actavis South Atlantic LLC, Sunrise, FL |
III |
Failed Dissolution Specifications: This recall is an extension of the recall initiated on 7/31/2013 for Bupropion HCI Extended-Release Tablets (XL) 300 mg, because another lot was shown to have similar failing results for dissolution at the 8-hour timepoint. |
|
Venlafaxine extended release Tablets, 225mg, 57,857 30- and 90-count bottles |
Osmotica Pharmaceutical Corp., Marietta, GA / Kremers Urban Pharmaceuticals, Princeton, NJ |
III |
Failed Dissolution Specification; 12 hour time point at 18 months shelf life. |