Flatiron Aims To Accelerate, Personalize Cancer Research
Executive Summary
Flatiron Health was formed to exploit a previously untapped source of real-world data on the treatment and clinical outcomes of cancer patients. The company is now working with most of the major players in the oncology sector as well as with the FDA to maximize the benefits of such data analytics.
- Flatiron Health's mission is to amass, organize and analyze clinical data relating to cancer patients who do not participate in clinical trials, and by doing so improve treatment and accelerate cancer research.
- The company offers a suite of tools to enhance patient outcomes and streamline operational efficiency in cancer treatment centers.
- Flatiron has also partnered with major pharmaceutical companies to use anonymized patient data to improve clinical trial design and to personalize treatments for patients.
It reads like a case study from a textbook on entrepreneurship. Two alumni of one of the US’ leading business schools, with a string of start-ups behind them, launch an advertising technology and software company that is later acquired by Google. While working at Google, both came to realize that, although the cancer landscape was evolving rapidly, the technology supporting research into new treatments was lagging far behind. Their response in 2012 was to create Flatiron Health Inc., a cancer analytics company whose stated mission is "serving cancer patients and our customers by dramatically improving treatment and accelerating research.”
Fast forward four years to May 2016 when Flatiron announced a research collaboration agreement with the US FDA to determine how anonymized data derived from patients not taking part in clinical trials can provide new insight into the safety and effectiveness of emerging anticancer therapies. As part of the agreement, Flatiron and the FDA are exploring the use of immunotherapeutic agents in patients with advanced non-small cell lung cancer (NSCLC), in particular analytic approaches, clinically relevant endpoints and methods for assessing safety. By early 2016, Flatiron had accrued data on nearly 30,000 NSCLC patients, of whom 1,500 patients were treated with immunotherapies – and that number has since grown to more than 4,000, far more patients than typically take part in clinical trials of new medications.
NSCLC was chosen as the first type of cancer to be studied as it was judged to offer the greatest potential impact on the most patients: the American Cancer Society put the number of new cases of lung cancer in 2015 in the US at 221,200, and the number of deaths at 158,040. NSCLC accounts for 83% of these cases.
The Flatiron/FDA collaboration followed the introduction of PD-1 inhibitors into clinical practice in the US. (PD-1, or programmed T-cell death 1, is a trans-membrane protein found on the surface of T cells that indirectly inhibits T-cell-mediated cytotoxicity.) In December 2014, the FDA approved Ono Pharmaceutical Co. Ltd.’s nivolumab (launched in the US for the treatment of NSCLC by Bristol-Myers Squibb Co. in January 2015, as Opdivo), whereas the indications for Merck & Co. Inc.’s Keytruda (pembrolizumab), already marketed in the US for the treatment of melanoma, were broadened in October 2015 to include NSCLC.
By July 2016, Flatiron had accrued data on 1,500 patients, and this number continues to grow – there are plans to expand the total to 30,000, far more patients than typically take part in clinical trials of new medications.
Amy Abernethy, MD, PhD, Flatiron’s chief medical officer and senior vice president, oncology, tells In Vivo that the FDA wanted to gain real-world evidence of the safety as well as the efficacy of PD-1 inhibitors via data pooled from individual cancer center records, as such evidence could not be derived from individual patient records. The outcome of this study will of course also be of great value to biopharmaceutical companies developing new cancer treatments. Abernethy says that Flatiron works with 11 out of the biggest 13 therapeutic oncology companies in the world as well as several smaller biotechnology companies.
In addition to the one for NSCLC, Flatiron is developing datasets for other significant cancers, and already has usable data for colon and breast cancer, melanoma, multiple myeloma and other malignancies. More patients and more data points are added each month, and the number of participating sites also continues to grow (data from more than 260 US cancer clinics and academic institutions representing around 1.5 million patients are currently in the system).
Google Origins
How did Flatiron come to occupy this leading position in the use of data analytics to improve cancer treatment? The company was founded in 2012 by Nat Turner and Zach Weinberg, who met as undergrads at the University of Pennsylvania's Wharton School. While still juniors at Wharton, Turner and Weinberg co-founded Invite Media, an advertising technology company based in New York City, which built the industry's first enterprise advertising platform for buying and optimizing online media in real time. Such was its success that Invite was acquired by Google in 2010, for a reported $81 million.
Abernethy says that Flatiron works with 11 out of the biggest 13 therapeutic oncology companies in the world as well as several smaller biotechnology companies.
Turner and Weinberg stayed on at Google for a couple of years to help integrate the Invite business. During that time, personal circumstances led the pair to turn their attention to the oncology sector. Noticing that the majority of cancer care centers, oncologists and researchers had limited or no access to even the most rudimentary data analysis tools, they founded Flatiron Health to address this deficiency. The name is taken from the location of the company’s offices in New York, across the street from the Flatiron Building (since November 2015 the company has also had an office in San Francisco).
Although treatments and patient outcomes during clinical trials are documented in detail, currently only some 4% of US cancer patients are actually enrolled in trials, which means that a lot of potentially useful data – from the other 96% of patients – is under-exploited, explains Abernethy. Turner, now the company's CEO and Weinberg, president and chief operating officer, realized that although the non-trial cancer patients' cases are documented in detail, the data generally remain in localized repositories, such as the records of individual cancer centers, and are thus not easily accessible to be aggregated and analyzed with the data from other patients. Part of the problem is that the way the information is stored is not consistent across the oncology sector. What was needed was a system that would permit all this siloed information to be pooled and analyzed on a common platform.
At the beginning of 2013, Flatiron raised $8 million in a Series A round of venture capital financing led by Google Ventures, with participation from First Round Capital, Laboratory Corp. of America Holdings (LabCorp), Great Oaks Capital, The Social+Capital Partnership, SV Angel, IA Ventures and a number of angel investors. As part of the financing, Krishna Yeshwant, MD, general partner at Google Ventures and a board member of Foundation Medicine Inc., joined Flatiron's board.
LabCorp, the only health care backer in the Series A round, won't comment on its specific interest in Flatiron. However, the company has a diverse portfolio of investments in diagnostic companies and in particular has a long-standing interest in the oncology area, launching its own oncology testing platform in the early 1990s.
Following the funding round, Flatiron was able to accelerate its product development, which was quickly followed by the first deployment of the OncoAnalytics tool in a cancer center, actually a small private oncology practice in Gettysburg, PA. Essentially, OncoAnalytics unlocks data from multiple systems in a manner consistent with the Health Insurance Portability and Accountability Act (HIPAA, which protects patient confidentiality), and delivers detailed clinical insights and business intelligence. By mid-2013, Flatiron was working with more than 10 cancer centers in the US, including universities and research centers. As noted, that figure currently stands at more than 260.
Structured Real-world Oncology Data And Intelligence
OncoAnalytics is part of Flatiron’s OncologyCloud suite of tools, which is designed to enhance patient outcomes and streamline operational efficiency in cancer treatment centers. Another component of the suite, OncoEMR, is an electronic health record that enables providers easily to document and manage patient care, and was acquired in May 2014 when Flatiron purchased Altos Solutions, a web-based oncology software company.
The OncologyCloud Suite
- OncoEMR, an electronic health record that enables cancer care providers to manage and document the entire treatment process within one cloud-based system;
- OncoAnalytics, a first-of-its-kind analytics tool that unlocks data from multiple systems and delivers detailed clinical insights and business intelligence;
- OncoBilling, an integrated system for filing and managing claims with insurance companies, designed particularly to work with OncoEMR; and
- SeeYourChart, a secure, cloud-based communications portal allowing patients to take an active role in their care, closely integrated with OncoEMR.
The Altos acquisition, which created what the company called in a press release “the world’s largest single source of structured real-world oncology data and intelligence,” coincided with a $130 million Series B venture capital round led by Google Ventures with the participation of First Round Capital and LabCorp.
The other components of OncologyCloud are OncoBilling, practice management and billing software designed to file and manage claims with payers, and SeeYourChart, a portal that allows patients to take an active role in their own care. The OncoBilling software permits the generation of financial reports and claims, review of claim status and printing of statements and receipts, and is claimed to facilitate processing and to save time. SeeYourChart is designed to help cancer centers to meet Meaningful Use requirements (and thus receive incentive payments) and allows providers to share clinical documents, lab results, appointment calendars and educational materials with patients.
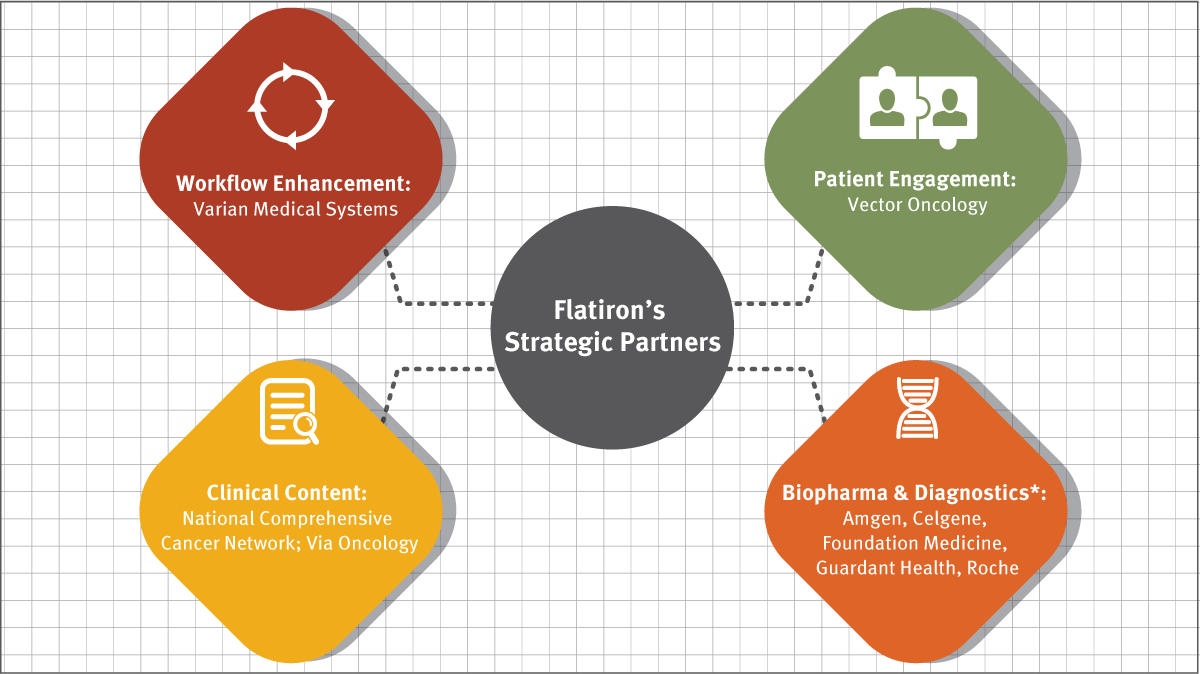
Another pivotal moment in Flatiron’s development came in May 2015, when the company announced a strategic alliance with Varian Medical Systems Inc. to develop a new generation of cloud-based, electronic health record, data analytics and decision support software for cancer care providers. For Flatiron, Varian was an ideal partner: not only is it a major supplier of radiotherapy, radiosurgery, proton therapy and brachytherapy systems for treating cancer patients, it also supplies informatics software for managing comprehensive cancer clinics, radiotherapy centers and medical oncology practices. The alliance was set up with the dual aims of enhancing patient outcomes and streamlining operational efficiency by offering a suite of cloud-based software solutions to support oncologists in delivering high-quality care across the cancer care spectrum, “from screening to survivorship,” as the two companies put it in a joint statement.
The first tangible outcome of the alliance was that both companies were able to offer their customers OncoEMR, which was made seamlessly interoperable with Varian’s ARIA OIS (oncology information system). The ARIA system combines radiation, medical and surgical oncology information into a complete, oncology-specific EMR.
Pharma Signs On
Flatiron closed a $175 million Series C round in January 2016. Roche was lead investor and was joined by Allen & Co, Baillie Gifford and Casdin Capital, among others. [See Deal]The equity stake entitled Roche to one seat on Flatiron’s board, with Daniel O’Day, CEO of Roche Pharmaceuticals, representing Roche. Flatiron, which does not disclose revenue or breakdown figures, says that it is now among the best capitalized start-ups in its category, and does not anticipate a need to seek further funding in the foreseeable future. It says its long-term goal is to remain an independent company, although it does not rule out a public flotation at some point.
Exhibit 1 Flatiron's Strategic Partnerships
*Partial list
Flatiron Health
At the same time as the Series C funding, Flatiron entered a multiyear, non-exclusive agreement with Roche under which the Swiss giant agreed to purchase a number of Flatiron’s life science offerings, with the two companies agreeing to collaborate to accelerate clinical trials, advance personalized medicine and enhance patient care.
Roche says that the collaboration will have benefits for both the development and commercial aspects of its business. Integrating real-world, clinical routine data and combining them with clinical trials data opens up promising opportunities, such as providing clues for new targets in R&D or optimizing treatment plans in the clinic. The combination of Flatiron’s data sets and analytics capabilities with Roche’s portfolio of innovative medicines and diagnostics expertise will help Roche drive productivity in R&D (with emphasis on clinical trial design and recruitment), increase access to innovative medicines and personalize treatments for patients for better outcomes, it claims.
In addition, Roche says it expects that Flatiron data will support its discussions with regulatory authorities, identify areas of unmet need, enable targeted patient enrollment in its oncology clinical trials and help to inform decisions critical for success, such as market planning.
Given the potential for conflict of interest, Flatiron maintains a strict firewall between Roche’s two roles as client and investor, Abernethy asserts. Its position as an investor does not mean that it obtains preferential treatment as a client, and strict confidentiality rules are observed in respect of Flatiron’s other clients.
Other participants in the Series C funding were Celgene Switzerland LLC and Amgen Ventures. In April 2016 Flatiron announced that, through individual agreements, Celgene Corp. and Amgen Inc. are to collaborate with Flatiron with the aim of accelerating clinical research, advancing personalized medicine and enhancing treatment options for cancer patients. For example, in addition to its equity investment, Celgene outlined plans to collaborate with Flatiron to utilize real-world evidence to identify novel approaches to patient therapy and to accelerate its aim of improving patients’ lives. Alan Colowick, MD, executive vice president at Celgene, says that the collaboration represents an ideal opportunity for the company to complement its scientific and clinical trial expertise with targeted insights from comprehensive real-world data. “Through our investment and relationship, Celgene expects to gain critical information from Flatiron’s unique platform that will be useful in our quest to discover and develop important therapies for patients,” he says.
For its part, Amgen says that it will leverage those of Flatiron’s products that integrate de-identified clinical treatment and outcomes data with its own genomic profiling data to accelerate clinical research efforts and inform the development of novel targeted therapies. Flatiron’s continued support of Amgen’s Oncology Services Comprehensive Electronic Records (OSCER) platform would assist its oncology teams in optimizing data for scientific research and enhance commercialization efforts, the company added. Elliott Levy, senior vice president, global development at Amgen, notes that the company has more than 10 years’ experience of capturing and analyzing oncology treatment data from electronic medical records, and that the collaboration with Flatiron “will help us take observational research to the next level.”
Flatiron does not have any third party payers among its clients even though a greater understanding of how anticancer drugs perform in the real world could potentially help inform formulary and other decisions.
Notably, while there is understandably much interest in Flatiron’s data from pharma companies developing new oncology treatments, Flatiron does not have any third party payers among its clients even though a greater understanding of how anticancer drugs perform in the real world could potentially help inform formulary and other decisions. Although the company has had discussions with payers on behalf of community oncology providers, it says its allegiance is with providers and enabling them to deliver safe, effective care in the most cost-efficient manner.
In November 2016, Flatiron announced that it is collaborating with Foundation Medicine to create a clinico-genomic database containing information on almost 20,000 patients. The HIPAA-compliant database combines genomic profiling data from patients whose tumors were sequenced with Foundation Medicine’s comprehensive genomic profiling (CGP) assays with annotated longitudinal clinical and outcomes data developed by Flatiron, and is intended to help researchers and biopharmaceutical companies accelerate the development of targeted therapeutics and immunotherapies for cancer.
The database is expected to grow in the coming months and is the first of several products that the two companies plan to co-develop. They are currently exploring partnerships within the research and academic communities to accelerate the use of the database for novel drug development and clinical trial applications.
Perhaps the last word on Flatiron’s activities should go to the World Economic Forum, which at a meeting in Tianjin, People’s Republic of China, in June 2016 named Flatiron as one of its Technology Pioneers. The honor is awarded to early-stage companies from around the world that are involved in the design, development and deployment of new technologies and innovations, and are poised to have a significant impact on business and society. In its citation, the WEF said that Flatiron “organizes the world’s oncology information to make it useful for patients, physicians, life science companies and researchers. Their software connects the oncology community on a common technology infrastructure to address key health care challenges. Ultimately, their mission is to serve cancer patients and members of the oncology community by dramatically improving treatment and accelerating research.” Previous recipients of the award include Airbnb, Google, Kickstarter, Mozilla, Spotify and Twitter.
Editor's note: this story was updated post-publication.