Device/Diagnostics Quarterly Deal Statistics, Q1 2015
Executive Summary
Device financing rose slightly to $934 million in Q1, while acquisition value was down to just over $4 billion. Diagnostics fundraising dropped significantly to $597 million; M&A activity was dominated by NGS and Roche's transaction with Foundation Medicine.
Device Transactions
Device financing reached $934 million during 2015's first quarter, a slight increase over Q4's $902 million. (See Exhibit 1.) Representing 37% of the financing total, follow-on public offerings drove most of the quarter's fundraising with $343 million, a significant increase over Q4's sole $12 million FOPO by implantable orthopedic device and biomaterials company Amedica Corp. [See Deal]
Cardiovascular device makerNeovasc Inc. raised the most in its $75 million FOPO. [See Deal] The Canadian company shares the transcatheter mitral valve implantation (TMVI) space with competitors including Edwards Lifesciences Corp. and CardiAQ Valve Technologies Inc. Neovasc is currently locked in a legal battle with CardiAQ, which alleges Neovasc is usingCardiAQ's TMVI technology (obtained in 2009 under a confidential agreement) in its pending European patent application for the Tiara valve.
IPOs were down from the previous quarter, but activity still remained strong with four deals totaling $189 million. Ear, nose, and throat (ENT) device maker Entellus Medical Inc. scored the most in an $84 million oversubscribed IPO priced at the top of its $15 to $17 range. [See Deal] This marks the highest IPO by an otolaryngology player. Besides Intersect ENT Inc.'s $59 million IPO last summer [See Deal], the last ENT device firm to go public was newborn screening company Natus Medical Inc., which also netted $59 million in its August 2001 offering. [See Deal]
The only transaction to hit the triple digits was Novocure Ltd.'s five-year $100 million term loan from Pharmakon Advisors. [See Deal] The company is developing the Tumor Treating Fields (TTFields) therapy, which uses electrical fields to treat brain cancer. Debt transactions overall represented 20% of the Q1 financing activity.
At $155 million, the venture funding category was just 17% of the first quarter's total (for 15 deals), less than half the $362 million that made up 40% (through 17 transactions) of the Q4 aggregate. Q1 late-stage transactions accounted for the majority of VC activity, led by heart and respiratory monitor developer EarlySense Ltd.’s $25 million Series F. [See Deal]
In all, financing by cardiovascular-focused companies made up $272 million, or 29%, of the quarter's financing. (See Exhibit 2.) They raised the greatest amount through follow-on offerings ($88 million), but the most popular financing vehicle in terms of number of deals was early-stage VC funding, bringing in $40 million.
Exhibit 2
Cardiovascular Players Bring In 29% Of Total Q1 2015 Financing
By Deal Type ($M)
NOTE: Chart doesn't include one debt deal that had an undisclosed value.
Strategic Transactions
The year's first quarter ended with a total of $4.1 billion in acquisitions of medical device players, down significantly from Q4 2014's $19 billion. (Over half the Q4 total, however, came from Becton Dickinson & Co.'s $11.8 million buy of medication management company CareFusion Corp. [See Deal]) Q1 2015's outliers were Cardinal Health Inc.'s acquisition of Johnson & Johnson's Cordis Corp. [See Deal] and Endo International PLC's divestiture of the men’s health business of its American Medical Systems Holdings Inc. division to Boston Scientific Corp. [See Deal], which at $1.9 billion, and $1.65 billion, respectively, together accounted for 86% of the Q1 total. (See Exhibit 3.)
Exhibit 3
Top Device M&As, Q1 2015
Strategic Transactions
|
Date |
Acquirer/Acquired (Business) |
Terms |
|
March |
Cardinal Health/Cordis (cardiac stents) |
$1.9B in cash; 2.49x sales |
|
March |
Boston Scientific/American Medical Systems' men's health business (urology) |
$1.65B: $1.6B up front, up to $50M in earn-outs; 4x sales |
|
February |
Nikon/Optos (ophthalmic digital imaging) |
$402M: $5.27 per share, a 32% premium; 2.36x sales |
|
February |
Globus Medical/Branch Medical (spinal and orthopedic implants and medical supplies) |
$52.9M in cash; 2.27x sales |
|
February |
Vision Acquisition/LCA-Vision (vision correction services) |
$40M in cash |
J&J's decision to exit the heart stent market![]() by divesting Cordis, allows Cardinal to capitalize on the company's international presence (about 70% of Cordis sales come from outside the US) and add the J&J division's cardiology and endovascular products, which Cardinal says are significantly preferred by physicians, but don’t differ much clinically from others in the same market segment. (See (Also see "Cardinal Health Builds On Generic Device Strategy With Cordis Deal" - Medtech Insight, 2 Mar, 2015.).) Cardinal first entered the US interventional cardiology space through its April 2014 buy of extravascular closure device company AccessClosure Inc. [See Deal], gaining its CE-marked and FDA-approved Mynx femoral artery sealing system, a product that complements the Cordis portfolio. However, according to Cardinal CEO Don Casey
by divesting Cordis, allows Cardinal to capitalize on the company's international presence (about 70% of Cordis sales come from outside the US) and add the J&J division's cardiology and endovascular products, which Cardinal says are significantly preferred by physicians, but don’t differ much clinically from others in the same market segment. (See (Also see "Cardinal Health Builds On Generic Device Strategy With Cordis Deal" - Medtech Insight, 2 Mar, 2015.).) Cardinal first entered the US interventional cardiology space through its April 2014 buy of extravascular closure device company AccessClosure Inc. [See Deal], gaining its CE-marked and FDA-approved Mynx femoral artery sealing system, a product that complements the Cordis portfolio. However, according to Cardinal CEO Don Casey![]() , rather than focusing solely on products, the company is instead centering its efforts on increasing its value by improving supply chain and distribution management processes and effecting cost efficiencies.
, rather than focusing solely on products, the company is instead centering its efforts on increasing its value by improving supply chain and distribution management processes and effecting cost efficiencies.
In a move Boston Scientific claims will position it as number one in the benign prostate hyperplasia, erectile dysfunction, and male stress urinary incontinence device markets (see (Also see "Boston Sci Ups Urology Focus With $1.6 Billion AMS Deal" - Medtech Insight, 2 Mar, 2015.)), it bought the men’s health business of AMS. The deal adds products in these areas to the existing offerings (in pelvic organ prolapse, kidney stones, female stress urinary incontinence, and abnormal uterine bleeding) of Boston Scientific's urology and women’s health segment. Boston Scientific believes these combined businesses could generate annual sales of about $1 billion. The current deal excludes AMS' women's health business, which is currently embroiled in lawsuits and regulatory inquiries involving safety issues related to its vaginal mesh implants. Endo itself acquired AMS (including both the men's and women's health care portfolios) for $2.9 billion in 2011. [See Deal]
Nikon Corp.'s acquisition of ophthalmic digital imaging company Optos PLC [See Deal] was one of the quarter's two transactions in vision care. The second was Vision Acquisition LLC's buy of vision correction services company LCA-Vision Inc. from PhotoMedex Inc. [See Deal] PhotoMedex divested LCA – which it had purchased for $103 million in February 2014 [See Deal] – to focus on its aesthetic and dermatology businesses.
Interestingly, the first quarter device alliance activity out-paced the M&As in deal volume. There were 18 alliances, while only 14 acquisitions were completed. The most active therapeutic areas for the licensing deals were cardiovascular, followed by wound healing. Cardio led with five transactions, including Boston Scientific's license of US distribution rights to CR Bard Inc.'s Lutonix 035 drug-coated balloon percutaneous transluminal angioplasty catheter. [See Deal] Q1 featured three partnerships involving wound healing devices, such as Arthrex Inc.'s gaining exclusive global rights to Vomaris Wound Care Inc.'s Procellera wound care technology. [See Deal]
Diagnostics Transactions
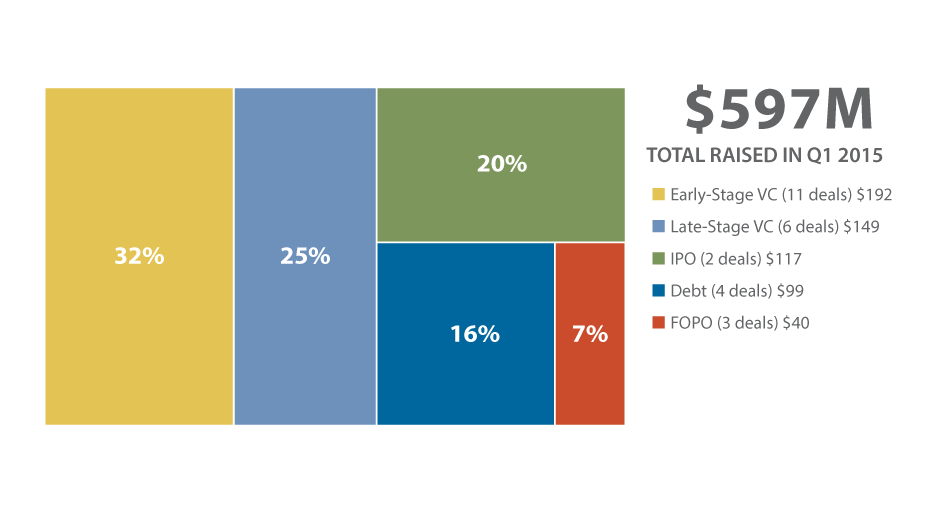
Diagnostic/research tools financing took a 20% nosedive in the first quarter of 2015, totaling $597 million, versus Q4 2014's $750 million. There were decreases in most of the fundraising types compared with last quarter's numbers; however, two categories experienced increases: IPOs and early-stage venture financing. (See Exhibit 4.)
While the number of IPOs only increased by one to two transactions in the first quarter, the total raised from the two companies – $117 million – was 20% of Q1's financing and a big jump over Great Basin Scientific Inc.'s lone $7.4 million Q4 2014 public offering. [See Deal] Most of that $117 million was attributed to Invitae Corp.'s $109 million float [See Deal], the largest financing of Q1 (Invitae was also the Q4 2014 leader with a $120 million Series F round [See Deal]). The five-year-old Genomic Health Inc. spin-off [See Deal] priced 7.3 million shares for $16, above its planned range. The firm provides DNA sequencing and genetic testing services focused on hereditary cancers. VolitionRx Ltd. completed the other Q1 IPO, netting $8.5 million on NYSE (it was previously listed on the OTCQB) to support its Nucleosomics biomarker technology to develop colorectal cancer immunoassays. [See Deal]
Early-stage venture rounds reached $192 million in Q1, making up the largest percentage – 32% – of the quarter's financing and more than 11 times the $17 million that was generated by this group in the previous quarter. Next-generation sequencing (NGS) company [10X Genomics Inc.]' $55.5 million Series B led the pack. [See Deal] Lead investor Foresite Capital Management was joined by Venrock, Paladin Capital Group, and Morgan Stanley Investment Management.
More than half of the 11 companies that raised early-stage funds focus on oncology (bringing in $51 million and representing 27% of the dollars), led by Lifecode Inc.'s $20.5 million Series A from Sequoia Capital, the Mayo Clinic, and Mayo Ventures. [See Deal] Using a variety of bioinformatics tools, Lifecode (formerly Silicon Valley Biosystems) just launched on a limited basis in the US its Pan Cancer Somatic Panel, which is conducted on solid tumors to aid in treatment decisions and monitoring.
Two early-stage fundraisers were boosted further by partnerships in Q1. Concurrently with closing a seven-digit Euro Series A round [See Deal], oncgnostics GMBH received limited nonexclusive global rights to MDxHealth SA's methylation-specific PCR technology, which oncgnostics will incorporate into its GynTect detection kit for cervical precancerous lesions. [See Deal] And a month after grossing $5.5 million in Series B financing [See Deal], Vigilant Biosciences Inc. partnered its OncAlert oral cancer risk assessment system with BL&H Co. Ltd. in Korea and Biotech-IgG AB in Denmark, Norway, and Sweden. [See Deal]
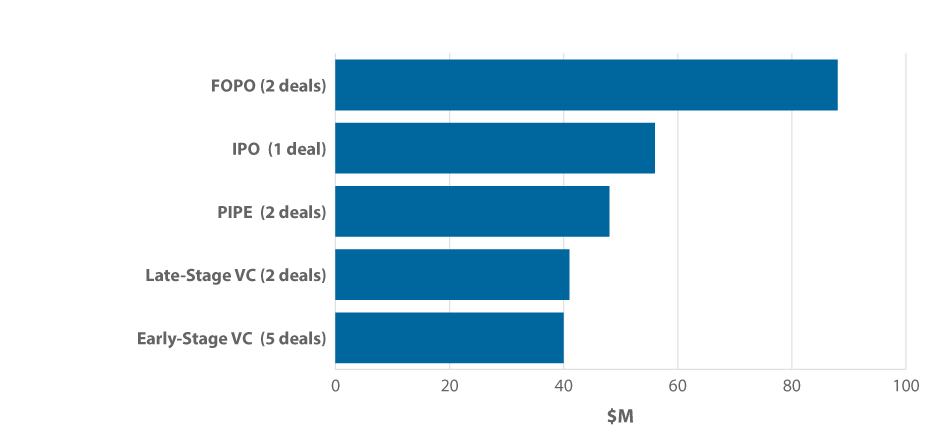
Lightspeed Venture Partners invested in the top two late-stage financings of the quarter, also done by cancer-focused entities. (See Exhibit 5.) Guardant Health Inc. brought in a $50 million Series C round to help gain more exposure for its Guardant360 biopsy-free test. [See Deal] Personalis Inc., which also completed a Series C financing ($33 million), offers off-the-shelf cancer genomics services. [See Deal]
Exhibit 5
Cancer Diagnostics Companies Reap Q1 2015 Venture Financing
SOURCE: Strategic Transactions
But in the late-stage group, which made up 25% of the quarter total at $149 million, there was also a strong showing of infectious disease firms, including backing from corporate investors. Novartis Venture Funds and Johnson & Johnson Development Corp. were among the buyers for Atlas Genetics Ltd.'s $20 million Series C round. [See Deal] Atlas hopes to soon launch its chlamydia test in Europe and start clinical development in the US. Merck Global Health Innovation Fund participated in a $15.5 million Series D round for Daktari Diagnostics Inc., which also plans to launch a test this year for HIV. [See Deal] And finally, Pocared Diagnostics Ltd. raised $15 million to finish clinical trials of its P-1000 analyzer that rapidly detects, identifies, and enumerates pathogens, including bacteria and yeast. [See Deal]
Nearly $1.6 billion went into diagnostics/research acquisitions during the first quarter, a slight increase over the Q4 2014's $1 billion. And a big part of these investments were in NGS. As was the case in the previous quarter, Roche again penned the largest deal in Q1, buying 57% of Foundation Medicine Inc. for just over $1 billion. [See Deal] (See Exhibit 6.) Under Roche's umbrella are now advanced capabilities in solid and liquid tumor genetic profiling via Foundation's FoundationOne and FoundationOne Heme. Many Big Pharmas are already using these technologies through collaborations with Foundation. The transaction drives toward Roche's goal of incorporating genomics into routine clinical testing. The transaction, which also includes a five-year R&D component involving molecular information and genomic analysis products for cancer [See Deal], is being compared with Roche's relationship with Genentech Inc. (See (Also see "Roche Gains Majority Stake In Foundation Medicine With R&D, Commercial Partnership" - Medtech Insight, 12 Jan, 2015.).)
Exhibit 6
Top Diagnostics M&As, Q1 2015
Strategic Transactions
|
Date |
Acquirer/Acquired (Business) |
Terms |
|
January |
Roche/Foundation Medicine (solid and liquid tumor genetic analysis tools) |
$1.05B: $50 per share (a 118% premium) for a 57% stake (on a fully diluted basis); 112.97x sales |
|
February |
Thermo Fisher/Advanced Scientifics (single-use bioprocessing equipment) |
$300M in cash; 3.75x sales |
|
January |
Qiagen NV/Enzymatics' Enzyme Solutions (next-generation sequencing reagents) |
$114M in cash |
|
January |
WuXi PharmaTech/NextCODE Health (sequence-based clinical diagnostics) |
$65M in cash |
|
January |
Abcam/Firefly BioWorks (multiplex assays, built using microfabrication technology, for biomarker detection) |
$28M in cash |
Foundation Medicine was the lone public company acquisition of Q1; the remaining 10 deals done during the quarter were for private firms, including Signature Diagnostics AG, also bought by Roche. [See Deal] Another boost to Roche's NGS efforts, Signature's biobank samples are combined with clinical progression and genetic data to form the basis of circulating cell-free DNA (cfDNA) tests. Signature will become part of Roche Diagnostics Corp.'s Roche Sequencing. (See "Roche underlines oncology goal with Signature buy,"![]() Clinica, February 10, 2015.)
Clinica, February 10, 2015.)
Another company involved in a pair of Q1 transactions was Qiagen NV, the Dutch producer of molecular diagnostics and NGS technologies. For $114 million Qiagen bought the Enzyme Solutions business of Enzymatics Inc. [See Deal], bringing Qiagen a portfolio of NGS reagents, which are used in over 80% of the world's NGS reactions. And management executives from Qiagen's Qiagen Marseille SA division bought out some assets to create HalioDx, which plans to advance an immunohistochemistry colon cancer test called Immunoscore licensed from French National Inst. Health & Med. Res. (INSERM). [See Deal]
In another notable Q1 NGS deal, Chinese drug and device discovery company WuXi PharmaTech Inc. paid $65 million for NextCODE Health, which has been renamed WuXi NextCODE Genomics. [See Deal] NextCODE was a 2013 spin off [See Deal] of deCODE genetics EHF, which has done pioneering work with genetics research in the Icelandic population, and was bought by Amgen Inc. in 2012. [See Deal] NextCODE, which provides approximately a 4.3x return to its investors through the WuXi acquisition, could significantly improve WuXi's genomic and bioinformatics products. In addition, WuXi says NextCODE's assets should complement work it's doing for Foundation Medicine in cancer genome decoding services in China. [See Deal] (See (Also see "NextCODE Buy Boosts WuXi’s Genomics Expertise" - Scrip, 13 Jan, 2015.).)