Entering The Generics Business In Mature Markets: Lessons Learned From 4,500 Launches
Executive Summary
In response to major market challenges, Big Pharmas have or are considering entering the small-molecule generics business. An IMS Consulting Group analysis of more than 4,500 generics launches between 2005 and 2010 tracks key attributes would-be branded company players need to know for success.
Many innovative pharmaceutical companies looking to diversify consider entering the small-molecule generics business, which they view as a logical adjacent space with comparatively low barriers to entry, and one that is synergetic with their existing business.
An IMS data-driven study of 4,500 generics launches confirms the importance of a strong local presence, large global scale, and a rapid launch following the originator’s loss of exclusivity as key determinants of a new generic’s uptake and eventual capture of volume share.
IMS warns that synergies between branded and generics businesses are less than assumed, the required investment can be considerable, and the risks can be high, especially for newcomers embarked on exploiting potentially lucrative, but unpredictable, Paragraph IV legal challenges in the US.
Faced with patent cliffs, constrained pipelines, and intensifying budget pressures, many R&D-driven large pharmaceutical companies are turning to non-proprietary products as a strategy for growth. They aim to expand into generics or over-the-counter products to retain the value of their own drugs after loss of exclusivity (LOE) and/or to capture additional cash flow from unprotected products that they either develop internally or in-license.
In many cases, these efforts center on exploring the opportunities inherent in marketing small-molecule generics. Companies view small-molecule generics as a logical adjacent space, because it seems to be a business that is relatively easy to enter, has synergies with current operations, and uses learnings already ingrained in their existing businesses. (See (Also see "Pharma: Serious About Change? " - In Vivo, 1 Oct, 2010.)and (Also see "North American Buyers Turn to European Branded Generics For Growth" - Pink Sheet, 6 Jun, 2011.).) But any entrant will face a highly mature, crowded, and fragmented market, dominated by pure-play generics companies with years of experience in the field, as well as by local legacy players that know how to survive in a tough environment. Research-based companies new to the game may not have the right skills, resources, and commitment; their capabilities and culture, designed to support innovation, are less suited to a commodity business based on volume.
While many Big Pharmas have already entered the field, they've done so with different levels of commitment and experience, mixed operating models, and varying degrees of success. (See (Also see "Sale Of Pfizer’s Established Products Not Off The Table, Exec Says" - Pink Sheet, 11 Sep, 2012.).) The challenges for new entrants raise a number of questions. What lessons can be learned from past market dynamics on how best to enter the generics business? How should companies structure their portfolio of products? What capabilities will they need? And how and when should they launch their products? (See (Also see "Abbott Established Products Senior VP Michael Warmuth Stresses Diversified Portfolio In Emerging Markets: An Interview With PharmAsia News (Part 1 of 2)" - Scrip, 19 Aug, 2011.)and (Also see "Abbott Established Products Senior VP Michael Warmuth Stresses Diversified Portfolio In Emerging Markets: An Interview With PharmAsia News (Part 2 of 2)" - Scrip, 22 Aug, 2011.).) To answer these questions, IMS Consulting Group studied the results of thousands of generic launches of "simple" oral solids in three mature generics markets (France, Germany, and the US), testing hypotheses about the circumstances under which launches were most successful. (See sidebar, “Lessons Learned From Generic Launches: About The Study.”)
As a result, we have identified the likely drivers of success and failure in entering mature generics markets.
Lesson 1: Build Local Strength
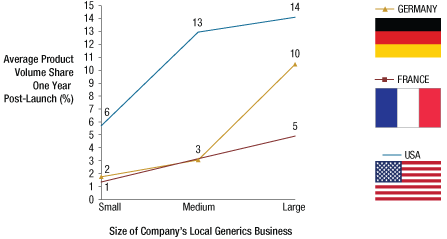
Strong local operations are particularly crucial for success in the generics market, which is typically highly fragmented with local, regional, and multinational players of various sizes competing within countries. Our study demonstrates that companies with a large generics presence in a given country command higher average volume shares per product. (See Exhibit 1.)
Exhibit 1
Product Share One-Year Post-Launch Correlates To Size Of Local Company’s Generics Business*
*Simple” orals only.
**Companies making up 50% of all generics sales in a country are defined as “large” for this study, the remaining companies generating between 50% and up to 80% of all generics sales are defined as “medium,” and all remaining companies are defined as “small.” According to this definition, in the US, France, and Germany, the top nine, eight, and 12 companies, respectively, are considered to be “large,” and the following 26, 22, and 41 companies, respectively, are “medium.”
IMS Consulting Group
Why Is This The Case?
Companies need large, local operations to achieve economies of scale in product marketing and distribution. A strong local presence also supports a detailed understanding of the local regulatory environment, solid relationships in the supply chain, and greater customer confidence in areas where generics have sometimes struggled, such as good manufacturing practice, supply continuity, and package quality. Such strengths are often cultivated over many years by dedicated teams with deep knowledge of the dynamics of the sector and the broader domestic environment.
Stay Up To Date On Generics
Click here![]() to receive an email alert when EBI publishes new articles on the generics industry.
to receive an email alert when EBI publishes new articles on the generics industry.
Lesson 2: Be Multinational
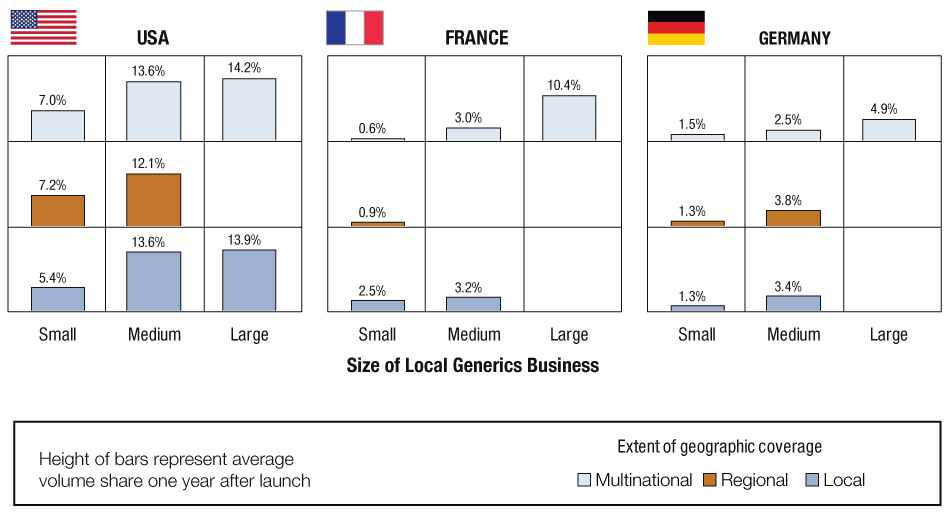
Geographic reach matters for generic companies, particularly in France and Germany, where all large players are multinational. Geographic reach is also increasingly important in the US, which traditionally has been large enough on its own to support economies of scale. Multinational companies with small local generics businesses did not benefit from their geographic reach independent of the size of their local branded business. (See Exhibit 2.)
Exhibit 2
Average Volume Share Of Generic Products One-Year Post-Launch*
*By size of company’s local generics business and extent of geographic coverage (“simple” oral solids only).
Local companies were defined as those with more than 80% of their sales in one country; regional companies as more than 80% of sales in three or fewer countries, and the remaining companies as multinational corporations.
IMS Consulting Group
Why Is This The Case?
Beyond being large enough to successfully market products in one country, generic companies also need to reach sufficient scale to justify investment in product development and registration, and to drive down costs in sourcing and manufacturing. Although the US market is big enough on its own, European players have to expand beyond borders to achieve synergies among several countries. However, being multinational only provides the scale needed to develop and price products competitively; local business size is the crucial factor in garnering a solid volume share.
What Are The Implications For New Players?
Companies committed to entering the space outside the US should expand their geographic footprint quickly to achieve the product volume required to justify the cost of development, registration, and manufacturing; however, companies must still build strength locally in important markets to achieve the synergies described in Lesson 1.
Lesson 3: Be First With New Products
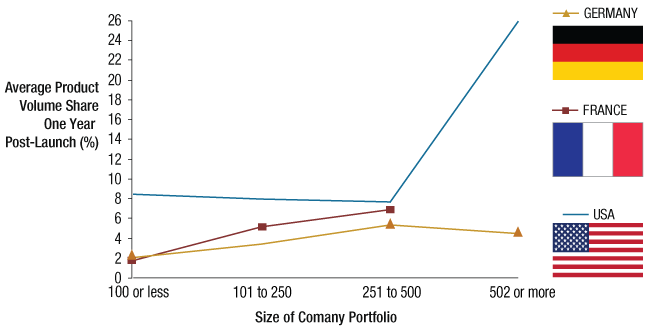
Companies with larger portfolios achieved a higher market share for their product launches in the US and France, with slightly lower share in Germany. (See Exhibit 3.)
Exhibit 3
Average Generic Product Volume Share By Size Of Company Portfolio One-Year Post-Launch*
* “Simple” oral solids only.
IMS Consulting Group
So, is a large portfolio of molecules the key to success? Not necessarily, with the exception of France, where, unlike in Germany or the US, independent pharmacies make the buying decisions for the bulk of generic prescription drug buyers. As a result, in France large portfolios are essential to justify large pharmacy sales forces.
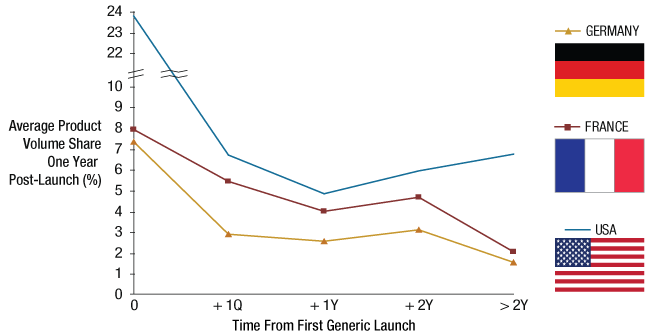
More significant is the timing of market entry. Successful companies in the generics space generally launched products near to the originator’s loss of exclusivity (LOE) and harvested returns generated by high-volume shares and prices, and moderately discounted pricing compared with the original innovator price. They then either kept these products or divested them, as the products faced more competition and became less profitable. (See Exhibit 4.)
Exhibit 4
Average Volume Share Of Generic Products One-Year Post-Launch Is Dependent On The Launch Timing*
*“Simple” oral solids only.
IMS Consulting Group
This advantage is similar or even higher than the average to be gained from focusing on more complex formulations. (See Exhibit 5.)
Exhibit 5
Average Product Volume Share One-Year Post-Launch By Product Form*
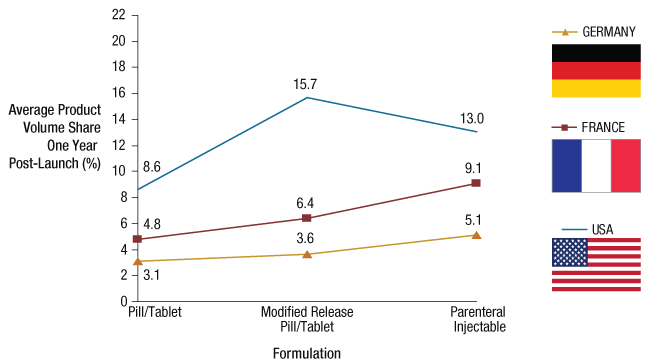
*“Simple” oral solids only.
IMS Consulting Group
In all three countries, first-to-market generics and first-wave entrants (Those launched within three months of the first entrant) maintained their first-mover advantage three years post-launch. (See Exhibit 6.)
Exhibit 6
Average Product Volume Share Over Time Of Generics Launching First*
*“Simple” oral solids only
IMS Consulting Group
What Are The Implications For New Players?
Although new products are the "cash cows" of the generics business, only an estimated total of 22 branded small molecules in the US and 15 in Germany are going off patent between 2013 and 2016. Launching old products in mature markets will provide small revenue streams due to lower shares and price levels. New players willing to face the odds should be prepared to commit resources to develop, in-license, or acquire new molecules, and bring them to market as a first generic.
Advice To Would-Be New Entrants
Incremental /Organic Growth Requires A Long-Term View
Building a generics business incrementally requires a mindset that is not inherent in research-based pharmaceutical companies. It is, therefore, often advisable to keep generics product development, sales, and marketing at arm’s length from core R&D business operations, and to invest in a separate structure bearing expertise in the field.
With the incremental growth option, companies must identify their portfolio early and invest in product development/licensing and regulatory activities years ahead of launch to bring first-mover generics to market. This requires a long-term view and many years of preparation and investment in the future organization and portfolio.
Synergies that can be achieved through partnerships and joint ventures will be relatively small, and players committed to being in this field should strongly consider acquiring generic companies with their portfolios, operations, and client base.
It May Not Be For You
Companies interested in the generics market primarily because parts of their portfolios are reaching the end of their lifecycle may want to consider alternative options. Proactive, product-lifecycle management may allow them to extract some value from off-patent brands, be it through novel formulations or launches in selected emerging markets where a brand adds incremental value to customers. Other alternatives include switching to over-the-counter status, head-on competition with generics on commercial terms in selective channels such as large tenders or through sick fund contracts, or making authorized generic deals with other players. Out-licensing and divestment of assets may also be attractive alternatives. In addition, for emerging markets where physicians remain key decision makers for branded generics usage, promoting selected in-licensed branded generics can be an option to complement local portfolios – however, such strategies are often driven by affiliates locally.
Play Big Or Stay Out
Major revenue streams justifying product development and registration come from products launched first – or at least early. In the US, companies must be prepared to make high-risk investments into Paragraph IV legal proceedings well before LOE if they aim to launch generics with an exclusivity period. Alternatively, more difficult-to-make products can offer an advantage to companies able to handle the complexities.
Yet, there are only a few, highly competitive opportunities on the horizon. Products with Paragraph IV filings will be difficult to acquire, and launching new generic versions of older, already registered molecules provides small returns.
If research-based companies want to build a substantial presence in generics in mature markets, they need to think big and make significant investments. They must be able to achieve scale in their generics distribution and promotion channels. This requires large, local generics revenue streams, country sales organizations tailored to generics channels and stakeholders, and years of experience. In Europe, multi-country presence is desirable to reach sufficient scale for manufacturing and dossier preparation (see (Also see "Generic Industry Specialization, Consolidation Driven By Rising Product Complexity" - Pink Sheet, 4 Mar, 2013.).)
In summary, success in the generics market is not a foregone conclusion, and R&D companies should invest heavily – or not at all.
Authors’ Note: We would like to thank John Cline and Alan Sheppard for their support with examples and hypotheses to test for this article.
Comments? Waseem Noor is a Vice-President at IMS Consulting Group in the New York Office. Stefan Lunglmayr is an Engagement Manager in the London Office. They are part of the Strategy & Portfolio Analysis practice and can be reached at [email protected]![]() and [email protected]
and [email protected]![]() .
.